IGCSE PHYSICS GRADE 11 TERM 1 ASSESSMENT BOOKLET
|
|
|
- Mitchell Adams
- 5 years ago
- Views:
Transcription
1 PHYSICS IGCSE PHYSICS GRADE 11 TERM 1 ASSESSMENT BOOKLET STS Page 1 of 44
2 PHYSICS PHYSI1101 ASSESSMENT TASK COVER PAGE Topic STS Performance Criteria Assessment event Date Time Thermal Physics 1.1 to to to to 4.6 Booklet for assessments Term1 Student Name Teacher Class Total Mark Grade 11 /20 I certify that the work presented is my own. Student signature: Date: Marked and feedback provided by: Signature: Date: Teacher comment: Student Comment: Feedback acknowledgement I acknowledge that I have received and understood feedback about this assessment. Student signature: STS Page 2 of 44
3 1. A student places his thumb firmly on the outlet of a bicycle pump, to stop the air coming out. 2. A balloon is inflated in a cold room. When the room becomes much warmer, the balloon becomes larger. 3. An engineer wants to fix a steel washer on to a steel rod. The rod is just too big to fit into the hole of the washer. STS Page 3 of 44
4 4. A substance is heated at a steady rate. It changes from a solid to a liquid, and then to a gas. 5. An experiment is set up to find out which metal is the best conductor of heat. Balls are stuck with wax to rods made from different metals, as shown in diagram X. STS Page 4 of 44
5 6. Thermometer X is held above an ice cube and thermometer Y is held the same distance below the ice cube. After several minutes, the reading on one thermometer changes. The ice cube does not melt. 7. Viewed through a microscope, very small particles can be seen moving with Brownian motion. Which line in the table is correct? 8. To mark a temperature scale on a thermometer, fixed points are needed. STS Page 5 of 44
6 9. A measured mass of gas is placed in a cylinder at atmospheric pressure and is then slowly compressed. 10. The graph shows the change in temperature of a material as it is heated. 11. Driving a car raises the temperature of the tyres. STS Page 6 of 44
7 12. An experiment is set up as shown. 13. An iron bar is held with one end in a fire. The other end soon becomes too hot to hold. STS Page 7 of 44
8 14. The diagram shows a block of ice placed in a warm room. 15. Four blocks, made of different materials, are each given the same quantity of internal (heat) energy. 16. A long thin bar of copper is heated evenly along its length. STS Page 8 of 44
9 17. A beaker contains water at room temperature. 18. Two plastic cups are placed one inside the other. Hot water is poured into the inner cup and a lid is put on top as shown. STS Page 9 of 44
10 19. A cylinder is filled with a gas and then sealed, so that the gas has a fixed volume. 20. Diagram 1 shows apparatus being used to observe smoke particles. STS Page 10 of 44
11 21. The graph shows how the temperature of hot liquid wax changes with time as the wax is allowed to cool kg of water and 1 kg of aluminium are heated to the same temperature and then allowed to cool in a room. 23. Bread can be cooked by placing it below, but not touching, a heating element. STS Page 11 of 44
12 24. The diagram shows a refrigerator. The cooling unit is placed at the top. The cooling unit cools the air near it. 25. Which line in the table describes the properties of solids and of liquids at a fixed temperature? 26. Air is pumped slowly into a car tyre to increase the pressure. The temperature of the air does not change. STS Page 12 of 44
13 27. The thermometer in the diagram has no scale. 28. A sample of a solid is heated for 12 minutes and its temperature noted every minute. STS Page 13 of 44
14 29. A heater is placed in a room. 30. The diagrams show four identical pieces of ice that are heated in test-tubes of water. STS Page 14 of 44
15 31. Which line in the table correctly describes whether the molecules of a solid, liquid and gas are moving or stationary? 32. Driving a car raises the temperature of the tyres. 33. The diagram shows how the atoms in a substance rearrange themselves during a change of state. STS Page 15 of 44
16 34. Equal masses of two different liquids are put into identical beakers. They are heated from 20 C to 30 C by heaters of the same power. Liquid 2 takes twice as long to heat as liquid There is a vacuum between the double walls of a vacuum flask. 36. Some water molecules escape from the surface of a lake. STS Page 16 of 44
17 37. The diagrams show four identical pieces of ice that are heated in test-tubes of water. 38. To mark the lower fixed point of a Celsius scale on a thermometer, the thermometer should be placed in STS Page 17 of 44
18 39. The diagram represents gas molecules contained in a cylinder. The piston is moved slowly downwards and the temperature of the gas stays the same. 40. A glass flask full of cool water is placed in a container of hot water. STS Page 18 of 44
19 41. A beaker of water is heated at its base. 42. A drop of liquid falls on a student s skin and quickly evaporates. 43. A suspension of pollen grains in water is observed under a microscope. The pollen grains are seen to be moving all the time. 44. A knife is being sharpened on a rotating sharpening-stone. A spark flies off and lands on the operator s hand. The spark is a very hot, very small piece of metal. The operator feels nothing. STS Page 19 of 44
20 45. Which substance is a liquid at a room temperature of 25 0 C? 46. The diagram shows a cooling unit in a refrigerator. 47. How does heat from the Sun reach the Earth? STS Page 20 of 44
21 48. A gas cylinder is left outside on a sunny day. 49. Water spilled on the ground on a hot day evaporates. 50. A block of ice is heated until it has all melted. The water that is produced is then heated until it boils. STS Page 21 of 44
22 51. A thermometer with no scale is taped to a ruler as shown. When placed in steam, the mercury level rises to 22 cm. When placed in pure melting ice, the mercury level falls to 2 cm. 52. Which line in the table is correct about conduction and convection? STS Page 22 of 44
23 53. A heating element is positioned in a narrow sealed tube of liquid. 54. The gas in a container is heated but is kept at constant volume. 55. The table lists the melting points and the boiling points of four different substances A, B, C and D. 56. The diagram shows four blocks of steel. The same quantity of heat is given to each block. STS Page 23 of 44
24 57. A wooden wheel can be strengthened by putting a tight circle of iron around it. 58. Which statement refers to convection? 59. Spoons made of different materials were placed in four cups of coffee poured from the same jug. STS Page 24 of 44
25 Theory questions 1. (a) Which of the following statements describe the property of a substance that would be suitable for measuring temperature? Tick the box alongside any acceptable statement. (b) Fig. 2.1 shows how the length of the thread in a liquid-in-glass thermometer varies with temperature. (i) What temperature is indicated by a thread length of 14.5 cm? temperature =... C (ii) What happens to the thread of the thermometer if the temperature drops below the ice point?... [2] STS Page 25 of 44
26 2. (a) State what is meant by the melting point of a solid. The melting point is......[2] (b) Which two of the following quantities are the same? Tick two boxes. (c) Some liquid in a beaker is kept boiling by heating the beaker, as shown in Fig (i) On the axes of Fig. 6.2, sketch a graph to show what happens to the temperature of the liquid whilst it is boiling. (ii) On your graph, mark the boiling point of the liquid. [2] STS Page 26 of 44
27 3. A thermocouple is used to measure the temperature of the inner wall of a pottery kiln. (a) In the space below, draw a labelled diagram of a thermocouple that could be used for this purpose. [2] (b) Describe (i) how you would read the temperature of the wall from the thermocouple, (ii) how the thermocouple works [2] (c) State two conditions in which a thermocouple is very suitable for temperature measurement [2] STS Page 27 of 44
28 4. (a) State two changes that usually happen to the molecules of a solid when the solid is heated [2] (b) Most substances expand when they are heated. (i) State one example where such expansion is useful.... [1] (ii) State one example where such expansion is a nuisance, and has to be allowed for.... [1] 5. (a) The table below describes the conditions of the molecules of a substance in each of the three states of matter, solid, liquid and gas. In the right-hand column, write the state of the substance that is described in the lefthand column. (b) (i) What is the state of matter just before a substance boils?... [1] (ii) Describe what happens to the molecules during boiling [2] STS Page 28 of 44
29 (iii) State two differences between boiling and evaporating [2] (c) (i) What is the state of matter just before a substance melts?... [1] (ii) Aluminium melts at 660 C. At what temperature does it freeze?... [1] 6. (a) Fig. 4.1 shows a cylinder containing air at a pressure of Pa. The length of the air column in the cylinder is 80 mm. The piston is pushed in until the pressure in the cylinder rises to Pa. Calculate the new length of the air column in the cylinder, assuming that the temperature of the air has not changed. (b) Fig. 4.2 shows the same cylinder containing air. new length =... [3] STS Page 29 of 44
30 The volume of the air in the cylinder changes as the temperature of the air changes. (i) The apparatus is to be used as a thermometer. Describe how two fixed points, 0 C and 100 C, and a temperature scale could be marked on the apparatus (ii) Describe how this apparatus could be used to indicate the temperature of a large beaker of water [5] 7. The air trapped in a cylinder by a piston is kept under pressure by a load, as shown in Fig STS Page 30 of 44
31 (a) Describe how the pressure in the cylinder is caused by the air molecules [3] (b) The load is increased. (i) State what happens to the piston.... (ii) State what happens to the pressure in the cylinder, and give a reason. what happens reason [3] 8. An immersion heater is put into some crushed ice at 0 C. The immersion heater is switched on. STS Page 31 of 44
32 (a) On Fig. 5.2, sketch the graph of temperature against time, up to the time when all the ice has melted. [3] (b) The heater is left switched on after all the ice has melted, and the temperature rises. After some time, the temperature stops rising, even though the heater is still on. (i) Suggest why the temperature stops rising, even though the heater is still on (ii) State what happens to the energy received by the water whilst this is happening [2] 9. (a) When a certain amount of heat is supplied to 1 kg of insulated aluminium, the temperature of the aluminium rises by 1 C. STS Page 32 of 44
33 In what form does the aluminium store the energy that has been supplied?...[1] (b) The same amount of heat is supplied to 1 kg of insulated copper, as shown in Fig The temperature rise of the 1 kg copper block is greater than the temperature rise of the 1 kg aluminium block in (a). Explain, in terms of thermal capacity, why this is so [2] 10. (a) Two identical open boxes originally contain the same volume of water. One is kept at 15 C and the other at 85 C for the same length of time. STS Page 33 of 44
34 Fig. 4.1 shows the final water levels. With reference to the energies of the water molecules, explain why the levels are different [3] (b) In an experiment to find the specific latent heat of vaporisation of water, it took J of energy to evaporate 15 g of water that was originally at 100 C. A second experiment showed that 600 J of energy was lost to the atmosphere from the apparatus during the time it took to evaporate 15 g of water. Calculate the specific latent heat of vaporisation of water that would be obtained from this experiment. specific latent heat = [3] 11. In a heating experiment, a student takes the temperature of a beaker B containing water at room temperature. Fig. 5.1 shows the thermometer used. STS Page 34 of 44
35 (a) State the temperature reading shown on the thermometer. temperature reading =... [1] (b) The student then transfers a small metal cylinder from beaker A of boiling water to the beaker B of water at room temperature, as shown in Fig The student assumes that the metal is at a temperature of 100 C when it enters the water in beaker B. The temperature of the water in beaker B rises to 36 C. (i) Calculate the temperature rise of the water in beaker B. (ii) Calculate the temperature fall of the metal cylinder. temperature rise =... temperature fall =... [3] (c) The student uses these readings and some other information to calculate the specific heat capacity of the metal. Why is it important to transfer the metal between the beakers as quickly as possible? [1] STS Page 35 of 44
36 12. Fig. 4.1 shows apparatus that a student uses to make an estimate of the specific heat capacity of iron. (a) The power of the heater is known. State the four readings the student must take to find the specific heat capacity of iron [3] (b) Write down an equation, in words or in symbols, that could be used to work out the specific heat capacity of iron from the readings in (a). [2] (c) (i) Explain why the value obtained with this apparatus is higher than the actual value [1] (ii) State one addition to the apparatus that would help to improve the accuracy of the value obtained [1] STS Page 36 of 44
37 13 Fig. 4.1 shows a student s attempt to estimate the specific latent heat of fusion of ice by adding ice at 0 C to water at 20 C. The water is stirred continuously as ice is slowly added until the temperature of the water is 0 C and all the added ice has melted. (a) Three mass readings are taken. A description of the first reading is given. Write down descriptions of the other two. reading 1 the mass of the beaker + stirrer + thermometer reading 2... reading 3... [2] (b) Write down word equations which the student could use to find (i) the heat lost by the water as it cools from 20 C to 0 C,... [1] (ii) the heat gained by the melting ice.... [1] (c) The student calculates that the water loses J and that the mass of ice melted is 30 g. Calculate a value for the specific latent heat of fusion of ice. STS Page 37 of 44
38 specific latent heat of fusion =... [2] (d) Suggest two reasons why this value is only an approximate value. Reason Reason [2] 14. (a) On a hot day, a child drinks all the water in a plastic bottle. She then screws the cap back tightly on the bottle, so that the bottle contains only air. She throws the bottle into a waste basket, where the Sun shines on it. After a while in the Sun s rays, the air in the bottle is much hotter than before. (i) State what has happened to the pressure of the air in the bottle.... (ii) In terms of the behaviour of the air molecules, explain your answer to (a)(i) [5] STS Page 38 of 44
39 (b) Also in the waste basket is a broken glass bottle containing a small quantity of water, as shown in Fig As the Sun shines on it, the volume of water slowly decreases. (i) State the name of the process causing this decrease.... (ii) In terms of the effect of the Sun s rays on the water molecules, explain your answer to (b)(i) [4] 15. The IGCSE class is investigating the change in temperature of hot water as cold water is added to it. The students are provided with 100 cm3 of hot water and a supply of cold water at room temperature. (a) The thermometer in Fig. 1.1 shows the temperature of the cold water. Record the temperature of the cold water, as shown in Fig [1] (b) A student records the temperature of the hot water. He then pours 20 cm3 of the cold water into the beaker containing the hot water. He records the temperature of the mixture of hot and cold water and the volume V of cold water added. He then repeats 3 the process four times until he has added a total of 100 cm of cold water. The table shows the readings. STS Page 39 of 44
40 (i) Complete the column headings in the table. [1] (ii) Use the data in the table to plot a graph of temperature _ (y-axis) against volume V (x-axis). STS Page 40 of 44
41 (c) A sketch graph of the readings taken by another student carrying out a similar experiment is shown in Fig The theoretical line shows the results expected by the student after calculating the values of. The student assumed that all the heat lost by the hot water was gained by the cold water when the cold water was poured into the beaker. The other line shows the experimental results. The student carried out the experiment with care. Suggest a practical reason why the experimental line differs from the theoretical line.... STS Page 41 of 44
42 ... [1] 16. Fig. 3.1 is an attempt to show the molecules in water and the water vapour molecules over the water surface. (a) Explain, in terms of the energies of the molecules, why only a few water molecules have escaped from the water surface [2] (b) State two ways of increasing the number of water molecules escaping from the surface [2] (c) Energy is required to evaporate water. Explain, in molecular terms, why this energy is needed STS Page 42 of 44
43 ... [2] 17. Fig. 5.1 shows a shallow dish containing a liquid that evaporates easily. The bulb of a thermometer is held in the liquid. A jet of air is blown over the surface of the liquid, so that the liquid evaporates rapidly. (a) State what happens to the reading shown on the thermometer....[1] (b) Explain your answer to (a) in terms of the behaviour of the molecules of the liquid [2] (c) State one example in everyday life where the effect demonstrated by this experiment occurs....[1] 18. Two identical open boxes originally contain the same volume of water. One is kept at 15 C and the other at 85 C for the same length of time. Fig. 4.1 shows the final water levels. STS Page 43 of 44
44 With reference to the energies of the water molecules, explain why the levels are different [3] Observations of a distant thunderstorm are made. STS Page 44 of 44
2 Thermal Physics. Thermal Physics. 1. Simple kinetic molecular model of matter 2. Thermal properties 3. Transfer of thermal energy
 2 Thermal Physics 1. Simple kinetic molecular model of matter 2. Thermal properties 3. Transfer of thermal energy 1. A swimmer climbs out of a swimming pool on a warm, dry day. Almost immediately he begins
2 Thermal Physics 1. Simple kinetic molecular model of matter 2. Thermal properties 3. Transfer of thermal energy 1. A swimmer climbs out of a swimming pool on a warm, dry day. Almost immediately he begins
MARIYA INTERNATIONAL SCHOOL AL-JUBAIL L-8 PHYSICS WORKSHEET(Heat & energy )
 MARIYA INTERNATIONAL SCHOOL AL-JUBAIL L-8 PHYSICS WORKSHEET(Heat & energy ) 1 Name... (a)fig. Shows a thermometer with a range of 10 C to 50 C. Explain what is meant by (i) the sensitivity of a thermometer,......
MARIYA INTERNATIONAL SCHOOL AL-JUBAIL L-8 PHYSICS WORKSHEET(Heat & energy ) 1 Name... (a)fig. Shows a thermometer with a range of 10 C to 50 C. Explain what is meant by (i) the sensitivity of a thermometer,......
UNIQUE SCIENCE ACADEMY
 1 (a) UNIQUE SIENE EMY Test (Unit 10-11) Name :... Paper: Physics ate :... lass: 1 Time llowed: 5Minutes Maximum Marks: 25 Theory Section: [Total 16 Marks] What do you understand by the term speccific
1 (a) UNIQUE SIENE EMY Test (Unit 10-11) Name :... Paper: Physics ate :... lass: 1 Time llowed: 5Minutes Maximum Marks: 25 Theory Section: [Total 16 Marks] What do you understand by the term speccific
ISLAMAYA ENGLISH SCHOOL
 1 (a) Some water is poured onto a plastic table-top, forming a puddle. The same volume of water is poured into a plastic dish, which is placed alongside the puddle. This is illustrated in Fig. 7.1. water
1 (a) Some water is poured onto a plastic table-top, forming a puddle. The same volume of water is poured into a plastic dish, which is placed alongside the puddle. This is illustrated in Fig. 7.1. water
liquid heating The density of the liquid changes as its temperature increases. This causes energy to be transferred throughout the liquid.
 1 liquid is heated in a beaker. liquid heating The density of the liquid changes as its temperature increases. This causes energy to be transferred throughout the liquid. How does the density change and
1 liquid is heated in a beaker. liquid heating The density of the liquid changes as its temperature increases. This causes energy to be transferred throughout the liquid. How does the density change and
I. C O N T E N T S T A N D A R D S
 Introductory Physics, High School Learning Standards for a Full First-Year Course I. C O N T E N T S T A N D A R D S and radiation between objects or regions that are at different temperatures. 3.1 Explain
Introductory Physics, High School Learning Standards for a Full First-Year Course I. C O N T E N T S T A N D A R D S and radiation between objects or regions that are at different temperatures. 3.1 Explain
UNIQUE SCIENCE ACADEMY
 1 (a) UNIQUE SIENE EMY Test (Unit 12) Name :... Paper: Physics ate :... lass: 1 Time llowed: 35Minutes Maximum Marks: 25 Theory Section: [Total 16 Marks] Fig. 1.1 shows a beaker in which coffee is served
1 (a) UNIQUE SIENE EMY Test (Unit 12) Name :... Paper: Physics ate :... lass: 1 Time llowed: 35Minutes Maximum Marks: 25 Theory Section: [Total 16 Marks] Fig. 1.1 shows a beaker in which coffee is served
UNIQUE COLLEGE OF COMPUTER SCIENCES General Certificate of Education Ordinary Level
 UNIQUE OLLEGE OF OMPUTER SIENES General ertificate of Education Ordinary Level PHYSIS Paper 1 Multiple hoice lass II2 III2 995054/11 January 2011 91 hour dditional Materials: Multiple hoice nswer Sheet
UNIQUE OLLEGE OF OMPUTER SIENES General ertificate of Education Ordinary Level PHYSIS Paper 1 Multiple hoice lass II2 III2 995054/11 January 2011 91 hour dditional Materials: Multiple hoice nswer Sheet
Unit THE NATURE OF HEAT
 Unit 5.0 - THE NATURE OF HEAT Heat is a form of energy, in the form of infrared radiation. Heat from the sun travels through space at the speed of 300,000,000 m/s. Upon arriving on earth, much of the radiant
Unit 5.0 - THE NATURE OF HEAT Heat is a form of energy, in the form of infrared radiation. Heat from the sun travels through space at the speed of 300,000,000 m/s. Upon arriving on earth, much of the radiant
Transfer of Heat. There are three ways in which heat is transferred from one body to another. These are
 Transfer of Heat In the precious chapter you have learnt that heat flows from a body at a higher temperature to a body at a lower temperature, but how does this transfer take place? You will find the answer
Transfer of Heat In the precious chapter you have learnt that heat flows from a body at a higher temperature to a body at a lower temperature, but how does this transfer take place? You will find the answer
A student investigated how much energy from the Sun was incident on the Earth s surface at her location.
 A student investigated how much energy from the Sun was incident on the Earth s surface at her location. She put an insulated pan of water in direct sunlight and measured the time it took for the temperature
A student investigated how much energy from the Sun was incident on the Earth s surface at her location. She put an insulated pan of water in direct sunlight and measured the time it took for the temperature
S2 Science. Heat. Homework Booklet
 S2 Science Heat Homework Booklet 1 1. The graph below shows how most substances cool after being taken away from the source of heat. Answer these questions about the graph a. where is the heat loss the
S2 Science Heat Homework Booklet 1 1. The graph below shows how most substances cool after being taken away from the source of heat. Answer these questions about the graph a. where is the heat loss the
Science 7. Unit 3. Heat and. Temperature
 Science 7 Unit 3 Heat and Temperature Name:_ Class:_ TOPIC 1 REINFORMCEMENT Putting Thermal Energy to Work Goal Develop ways to classify natural and manufactured structures. BLM 3-1 When do you use thermal
Science 7 Unit 3 Heat and Temperature Name:_ Class:_ TOPIC 1 REINFORMCEMENT Putting Thermal Energy to Work Goal Develop ways to classify natural and manufactured structures. BLM 3-1 When do you use thermal
Thermal Properties and Temperature
 Thermal Properties and Temperature Question Paper 5 Level IGCSE Subject Physics Exam Board CIE Topic Thermal Physics Sub-Topic Thermal Properties and Temperature Paper Type Alternative to Practical Booklet
Thermal Properties and Temperature Question Paper 5 Level IGCSE Subject Physics Exam Board CIE Topic Thermal Physics Sub-Topic Thermal Properties and Temperature Paper Type Alternative to Practical Booklet
HEAT What are some sources of Heat?
 HEAT Activity 1. Rub your hands quickly against each other. 2. Then quickly place them onto your face. 3. Describe what you feel. Your hands feel warm. 4. Why does it feel warm? It feels warm as HEAT has
HEAT Activity 1. Rub your hands quickly against each other. 2. Then quickly place them onto your face. 3. Describe what you feel. Your hands feel warm. 4. Why does it feel warm? It feels warm as HEAT has
St. Anthony's Canossian Secondary School Sec 3E Science (Physics) Chapter 9 Transfer of Thermal Energy. Name: ( ) Class: Sec Date:
 St. Anthony's Canossian Secondary School Sec 3E Science (Physics) Chapter 9 Transfer of Thermal Energy Name: ( ) Class: Sec Date: Candidates should be able to: (a) show understanding that thermal energy
St. Anthony's Canossian Secondary School Sec 3E Science (Physics) Chapter 9 Transfer of Thermal Energy Name: ( ) Class: Sec Date: Candidates should be able to: (a) show understanding that thermal energy
Science Test Revision
 John Buchan Middle School Science Test Revision 5D Changing State 52 min 50 marks Name John Buchan Middle School 1 Level 3 1. Changes (a) Kim and Juan change the way some things look. The pictures below
John Buchan Middle School Science Test Revision 5D Changing State 52 min 50 marks Name John Buchan Middle School 1 Level 3 1. Changes (a) Kim and Juan change the way some things look. The pictures below
Changes of State. Lesson 1
 Lesson 1 Changes of State If all the ice in the world melted, the oceans would rise by more than 65 meters (215 feet)! This iceberg is melting in Paraiso Bay, Antarctica. What happens to ice when it melts?
Lesson 1 Changes of State If all the ice in the world melted, the oceans would rise by more than 65 meters (215 feet)! This iceberg is melting in Paraiso Bay, Antarctica. What happens to ice when it melts?
3B Heat, Light and Sound
 Heat, Light and Sound Heat, light and sound are forms of energy that have many applications in our lives. Heat Heat is a form of energy When petrol burns in a car engine it causes the gases in the cylinders
Heat, Light and Sound Heat, light and sound are forms of energy that have many applications in our lives. Heat Heat is a form of energy When petrol burns in a car engine it causes the gases in the cylinders
VISUAL PHYSICS ONLINE THERMODYNAMICS WHAT HAPPENS WHEN SOMETHING IS HEATED? LATENT HEAT
 VISUAL PHYSICS ONLINE THERMODYNAMICS WHAT HAPPENS WHEN SOMETHING IS HEATED? LATENT HEAT 1 HEATS OF FUSION AND VAPORISATION From the equation containing the specific heat, it seems that we can keep on transferring
VISUAL PHYSICS ONLINE THERMODYNAMICS WHAT HAPPENS WHEN SOMETHING IS HEATED? LATENT HEAT 1 HEATS OF FUSION AND VAPORISATION From the equation containing the specific heat, it seems that we can keep on transferring
In Chapter 3 you learnt that woollen
 4 Heat In Chapter 3 you learnt that woollen clothes are made from animal fibres. You also know that cotton clothes are made from plant fibres. We wear woollen clothes during winters when it is cold outside.
4 Heat In Chapter 3 you learnt that woollen clothes are made from animal fibres. You also know that cotton clothes are made from plant fibres. We wear woollen clothes during winters when it is cold outside.
More heat energy means more of what type of energy? Does the mass change? So, what must change? What is the same in both containers?
 Quest Chapter 21-23 # Problem Hint 1 When a container of gas is heated, what happens to the average speed of its molecules? 1. Additional information is needed. 2. increases 3. doesn t change 4. decreases
Quest Chapter 21-23 # Problem Hint 1 When a container of gas is heated, what happens to the average speed of its molecules? 1. Additional information is needed. 2. increases 3. doesn t change 4. decreases
Heat and temperature. Making a thermometer
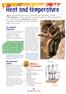 Heat and temperature A bout four hundred years ago, it would have been impossible to tell the temperature of the air, the water or any other substance. That s because there was no such thing as a thermometer
Heat and temperature A bout four hundred years ago, it would have been impossible to tell the temperature of the air, the water or any other substance. That s because there was no such thing as a thermometer
Activity Sheet Chapter 1, Lesson 1 Molecules Matter
 Activity Sheet Chapter 1, Lesson 1 Molecules Matter Name: Date: Question to investigate- Is the speed of water molecules different in hot and cold water? Hot water in a clear plastic cup Cold water in
Activity Sheet Chapter 1, Lesson 1 Molecules Matter Name: Date: Question to investigate- Is the speed of water molecules different in hot and cold water? Hot water in a clear plastic cup Cold water in
7 In the process of convection, heat energy is transferred C D E. 9 Boiling water and ice can exist at the same time in a test tube.
 TOPI 9 Transfer of Thermal Energy 1 How may heat be transferred through a vacuum? by convection only by radiation only by conduction only by convection and radiation only E by conduction. convection and
TOPI 9 Transfer of Thermal Energy 1 How may heat be transferred through a vacuum? by convection only by radiation only by conduction only by convection and radiation only E by conduction. convection and
(a) (i) Through which part of the house is most heat lost? How can the heat loss through the windows be reduced? ...
 Q1. The diagram shows where heat is lost from a house that is not insulated. (a) (i) Through which part of the house is most heat lost? (ii) How can the heat loss through the windows be reduced? (b) A
Q1. The diagram shows where heat is lost from a house that is not insulated. (a) (i) Through which part of the house is most heat lost? (ii) How can the heat loss through the windows be reduced? (b) A
Fundamentals of Heat Transfer
 Fundamentals of Heat Transfer State basic laws of heat transfer Estimate heat transfer rates by different modes Use the concept of thermal resistance to solve steady state heat transfer problems State
Fundamentals of Heat Transfer State basic laws of heat transfer Estimate heat transfer rates by different modes Use the concept of thermal resistance to solve steady state heat transfer problems State
Activity Sheet Chapter 2, Lesson 1 Heat, Temperature, and Conduction
 Activity Sheet Chapter 2, Lesson 1 Heat, Temperature, and Conduction Name Date In this activity, you will place a room-temperature set of washers in hot water and then place a set of hot washers in room-temperature
Activity Sheet Chapter 2, Lesson 1 Heat, Temperature, and Conduction Name Date In this activity, you will place a room-temperature set of washers in hot water and then place a set of hot washers in room-temperature
Heat can change into other forms of energy and vice versa. Heat is measured in the unit of energy, the joule (J).
 ExamLearn.ie Heat Heat Heat has the ability to do work or move something. Thus, heat is a form of energy. Heat can change into other forms of energy and vice versa. Heat is measured in the unit of energy,
ExamLearn.ie Heat Heat Heat has the ability to do work or move something. Thus, heat is a form of energy. Heat can change into other forms of energy and vice versa. Heat is measured in the unit of energy,
St. Anthony's Canossian Secondary School Sec 3NA Science (Physics) Chapter 7 Transfer of Thermal Energy. Name: ( ) Class: Sec Date:
 St. Anthony's Canossian Secondary School Sec 3NA Science (Physics) Chapter 7 Transfer of Thermal Energy Name: ( ) Class: Sec Date: Candidates should be able to: (a) show understanding that thermal energy
St. Anthony's Canossian Secondary School Sec 3NA Science (Physics) Chapter 7 Transfer of Thermal Energy Name: ( ) Class: Sec Date: Candidates should be able to: (a) show understanding that thermal energy
Estimate the energy stored in unit gram of the food in J per gram.
 NAME : F.5 ( ) Marks: /70 FORM FIVE PHYSICS REVISION TEST on HEAT Time Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section
NAME : F.5 ( ) Marks: /70 FORM FIVE PHYSICS REVISION TEST on HEAT Time Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section
Thermal Energy Worksheets
 Thermal Energy Worksheets Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
Thermal Energy Worksheets Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
When both switches are on, the heater works at the high power setting. What is the power of the heater when it is switched to the high power setting?
 1 (a) The diagram shows two switches on a room heater. The heater has three power settings. The power produced by two of the settings is given in the table. Setting Power in kw Low 0.5 Medium 1.5 High
1 (a) The diagram shows two switches on a room heater. The heater has three power settings. The power produced by two of the settings is given in the table. Setting Power in kw Low 0.5 Medium 1.5 High
Q1. The diagram shows an experiment to find out what happens to infrared waves when they strike different surfaces.
 Q1. The diagram shows an experiment to find out what happens to infrared waves when they strike different surfaces. (a) The water in the black tube gets hotter than the water in the shiny tube. Choose
Q1. The diagram shows an experiment to find out what happens to infrared waves when they strike different surfaces. (a) The water in the black tube gets hotter than the water in the shiny tube. Choose
Particle Model Questions 1
 Particle Model Questions 35 Questions Name: Class: Date: Time: Marks: Comment s: Q. Figure shows solid ice on a car s rear window. Figure Captive cookies/istock/thinkstock The glass window contains an
Particle Model Questions 35 Questions Name: Class: Date: Time: Marks: Comment s: Q. Figure shows solid ice on a car s rear window. Figure Captive cookies/istock/thinkstock The glass window contains an
Changes of phase usually involve a transfer of energy Evaporation
 Changes of phase usually involve a transfer of energy. The four possible forms of matter solid, liquid, gas, and plasma are called phases. Matter can change from one phase to another. The phase of matter
Changes of phase usually involve a transfer of energy. The four possible forms of matter solid, liquid, gas, and plasma are called phases. Matter can change from one phase to another. The phase of matter
Different energy sources can be used to generate electricity.
 Q1. Electricity is a useful form of energy. (a) Different energy sources can be used to generate electricity. Give one advantage and one disadvantage (other than cost) of using each energy source to generate
Q1. Electricity is a useful form of energy. (a) Different energy sources can be used to generate electricity. Give one advantage and one disadvantage (other than cost) of using each energy source to generate
Thermal Energy Study Guide
 Goal 1 Thermal Energy Study Guide Name: Hour Define the following in your own words: 1. Conductor - a material that allows heat to flow through it easily Two examples - metal pot, fork 2. Insulator - a
Goal 1 Thermal Energy Study Guide Name: Hour Define the following in your own words: 1. Conductor - a material that allows heat to flow through it easily Two examples - metal pot, fork 2. Insulator - a
Activity Heat Transfer
 Name: Hr: Activity 3.3.6 Heat Transfer Introduction Heat is the transfer of thermal energy. Thermal energy exists when the atoms or molecules in a substance are in motion and vibrate. The atoms possess
Name: Hr: Activity 3.3.6 Heat Transfer Introduction Heat is the transfer of thermal energy. Thermal energy exists when the atoms or molecules in a substance are in motion and vibrate. The atoms possess
Fundamentals of Heat Transfer
 Fundamentals of Heat Transfer State basic laws of heat transfer Estimate heat transfer rates by different modes Use the concept of thermal resistance to solve steady state heat transfer problems State
Fundamentals of Heat Transfer State basic laws of heat transfer Estimate heat transfer rates by different modes Use the concept of thermal resistance to solve steady state heat transfer problems State
NAME:... HEATING EFFECT OF ELECTRIC CURRENT. Page 1
 NAME:... HEATING EFFECT OF ELECTRIC CURRENT www.kcpe-kcse.com Page 1 1. A car heater has two identical heating elements. The car battery can send 15000 C through the circuit in an hour. (i) What is the
NAME:... HEATING EFFECT OF ELECTRIC CURRENT www.kcpe-kcse.com Page 1 1. A car heater has two identical heating elements. The car battery can send 15000 C through the circuit in an hour. (i) What is the
ST. GABRIEL S SECONDARY SCHOOL Lower Secondary Science Chapter 7 Transfer of Thermal Energy
 ST. GRIEL S SEONRY SHOOL Lower Secondary Science hapter 7 Transfer of Thermal Energy Worksheet 7.1 Name : _Suggested nswers ( ) lass : ate : Section : Multiple hoice Questions 1 Two similarly-sized blocks,
ST. GRIEL S SEONRY SHOOL Lower Secondary Science hapter 7 Transfer of Thermal Energy Worksheet 7.1 Name : _Suggested nswers ( ) lass : ate : Section : Multiple hoice Questions 1 Two similarly-sized blocks,
Describe the movement of the particles of helium gas inside the balloon (2)
 The figure below shows a balloon filled with helium gas. (a) Describe the movement of the particles of helium gas inside the balloon............. (2) (b) What name is given to the total kinetic energy
The figure below shows a balloon filled with helium gas. (a) Describe the movement of the particles of helium gas inside the balloon............. (2) (b) What name is given to the total kinetic energy
Section 9. Comparing Energy Consumption: More for Your Money. What Do You See? What Do You Think? Investigate. Learning Outcomes
 Section 9 Comparing Energy Consumption: More for Your Money Section 9 Comparing Energy Consumption: More for Your Money What Do You See? Learning Outcomes In this section, you will Measure and compare
Section 9 Comparing Energy Consumption: More for Your Money Section 9 Comparing Energy Consumption: More for Your Money What Do You See? Learning Outcomes In this section, you will Measure and compare
THERMODYNAMICS PREPARED BY DW & JD
 THERMODYNAMICS PREPARED BY DW & JD 1 HEAT FLOWS QUESTION: heat flows in 3 ways what are they? 2 HEAT FLOWS ANSWER: 1. CONDUCTON 2. CONVECTION 3. RADIATION 3 HEAT FLOWS CONDUCTION: the transfer of heat
THERMODYNAMICS PREPARED BY DW & JD 1 HEAT FLOWS QUESTION: heat flows in 3 ways what are they? 2 HEAT FLOWS ANSWER: 1. CONDUCTON 2. CONVECTION 3. RADIATION 3 HEAT FLOWS CONDUCTION: the transfer of heat
Q1. The diagram shows the design of a solar cooker. The cooker heats water using infrared radiation from the Sun.
 Q. The diagram shows the design of a solar cooker. The cooker heats water using infrared radiation from the Sun. (a) Why is the inside of the large curved dish covered with shiny metal foil? () (b) Which
Q. The diagram shows the design of a solar cooker. The cooker heats water using infrared radiation from the Sun. (a) Why is the inside of the large curved dish covered with shiny metal foil? () (b) Which
energy from hot flame [3] [2]
![energy from hot flame [3] [2] energy from hot flame [3] [2]](/thumbs/79/79286354.jpg) 1 This question is about energy transfer and how it is used in cooking. (a) Steve heats a pan of water on his cooker. Look at the diagram. water aluminium base energy from hot flame (i) Explain how the
1 This question is about energy transfer and how it is used in cooking. (a) Steve heats a pan of water on his cooker. Look at the diagram. water aluminium base energy from hot flame (i) Explain how the
UNIQUE SCIENCE ACADEMY
 UNIQUE SIENE EMY Test (Unit 11-12) Name :... Paper: Physics ate :... ode: 5054 lass: II Time llowed: 35Minutes This document consists of 5 printed pages. Maximum Marks: 25 T [Total 21 Marks] heory Section:
UNIQUE SIENE EMY Test (Unit 11-12) Name :... Paper: Physics ate :... ode: 5054 lass: II Time llowed: 35Minutes This document consists of 5 printed pages. Maximum Marks: 25 T [Total 21 Marks] heory Section:
Describe the movement of the particles of helium gas inside the balloon
 Q1.The figure below shows a balloon filled with helium gas. (a) Describe the movement of the particles of helium gas inside the balloon............. (b) What name is given to the total kinetic energy and
Q1.The figure below shows a balloon filled with helium gas. (a) Describe the movement of the particles of helium gas inside the balloon............. (b) What name is given to the total kinetic energy and
Directions: Read/complete the following sections on the Transfer of Thermal Energy
 Name: _ Period: Date: _ Notes: Thermal Energy Transfers Directions: Read/complete the following sections on the Transfer of Thermal Energy Conduction Heat is transmitted by conduction, convection and radiation.
Name: _ Period: Date: _ Notes: Thermal Energy Transfers Directions: Read/complete the following sections on the Transfer of Thermal Energy Conduction Heat is transmitted by conduction, convection and radiation.
THERMAL CONDUCTION. placed in a different position. Can you explain why the matches go out?
 THERMAL CONDUCTION NAME(S) Pour about 250 ml of water into a 500 ml beaker, and begin heating the beaker on a hot plate. The beaker of water will be used in a later activity. Activity #1 A Parlor Trick
THERMAL CONDUCTION NAME(S) Pour about 250 ml of water into a 500 ml beaker, and begin heating the beaker on a hot plate. The beaker of water will be used in a later activity. Activity #1 A Parlor Trick
PHYSICS FORM 5 TRANSFER OF THERMAL ENERGY
 Heat energy is transferred from one place to the next by three mechanisms: 1. Conduction 2. Convection 3. Radiation Conduction This is the process of heat transfer from one place to another using the movement/vibration
Heat energy is transferred from one place to the next by three mechanisms: 1. Conduction 2. Convection 3. Radiation Conduction This is the process of heat transfer from one place to another using the movement/vibration
NCERT solution for Heat
 NCERT solution for Heat 1 Question 1 State similarities and differences between the laboratory thermometer and the clinical thermometer. Similarities Both of them are made up of uniform glass tube Differences
NCERT solution for Heat 1 Question 1 State similarities and differences between the laboratory thermometer and the clinical thermometer. Similarities Both of them are made up of uniform glass tube Differences
Conduction, Convection, and Radiation Lab Page 1 of 5
 Conduction, Convection, and Radiation Lab Page 1 of 5 I. Objectives 1. Explain the differences among conduction, convection, and radiation. 2. Investigate thermal conductivity in common metals, convection
Conduction, Convection, and Radiation Lab Page 1 of 5 I. Objectives 1. Explain the differences among conduction, convection, and radiation. 2. Investigate thermal conductivity in common metals, convection
Infra-Red Radiation. Question Paper. Save My Exams! The Home of Revision. Exam Board. Page 1. Score: /68. Percentage: /100
 Infra-Red Radiation Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Physics AQA P1 Infra-Red Radiation Silver Level Question Paper Time Allowed: 68 minutes Score: /68 Percentage:
Infra-Red Radiation Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Physics AQA P1 Infra-Red Radiation Silver Level Question Paper Time Allowed: 68 minutes Score: /68 Percentage:
Keep It Hot! Handout
 Keep It Hot! Handout Day 1: Introduction Today, you will study heat transfer by conduction and convection! Fill in the rest of these statements: Heat is Heat always flows from to If we heat up a material
Keep It Hot! Handout Day 1: Introduction Today, you will study heat transfer by conduction and convection! Fill in the rest of these statements: Heat is Heat always flows from to If we heat up a material
Level 1 Physics, 2016
 90939 909390 1SUPERVISOR S Level 1 Physics, 2016 90939 Demonstrate understanding of aspects of heat 2.00 p.m. Tuesday 15 November 2016 Credits: Four Achievement Achievement with Merit Achievement with
90939 909390 1SUPERVISOR S Level 1 Physics, 2016 90939 Demonstrate understanding of aspects of heat 2.00 p.m. Tuesday 15 November 2016 Credits: Four Achievement Achievement with Merit Achievement with
What percentage of infra red is absorbed by the glass? increases. does not change. decreases. Blacksurfaces are poor emitters of infra red radiation.
 Q1. (a) Infra red radiation can be reflected, absorbed and transmitted by glass. (i) What percentage of infra red is absorbed by the glass?... (ii) Complete the following sentence by drawing a ring around
Q1. (a) Infra red radiation can be reflected, absorbed and transmitted by glass. (i) What percentage of infra red is absorbed by the glass?... (ii) Complete the following sentence by drawing a ring around
The equation which links current, potential difference and resistance is:
 Q1.An electrical circuit is shown in the figure below. (a) The current in the circuit is direct current. What is meant by direct current? Tick one box. Current that continuously changes direction. Current
Q1.An electrical circuit is shown in the figure below. (a) The current in the circuit is direct current. What is meant by direct current? Tick one box. Current that continuously changes direction. Current
5. Transfer of thermal energy
 5. Transfer of thermal energy A bird can reduce the heat loss from its body during cold weather by fluffing up its feathers. What are the processes of heat transfer? Conduction Convection Radiation transfer
5. Transfer of thermal energy A bird can reduce the heat loss from its body during cold weather by fluffing up its feathers. What are the processes of heat transfer? Conduction Convection Radiation transfer
S water = J/gC
 EXPERIMENT 7 Specific Heat Capacity of A Metal INTRODUCTION One of the properties of matter is that it requires a certain amount of heat energy to raise the temperature of a unit mass of a substance. This
EXPERIMENT 7 Specific Heat Capacity of A Metal INTRODUCTION One of the properties of matter is that it requires a certain amount of heat energy to raise the temperature of a unit mass of a substance. This
LESSON CLUSTER 9 Explaining Condensation and the Water Cycle
 LESSON CLUSTER 9 Explaining Condensation and the Water Cycle Lesson 9.1: Boiling and Condensation You have been studying changes of state for quite a while now. You have studied melting, freezing or solidifying,
LESSON CLUSTER 9 Explaining Condensation and the Water Cycle Lesson 9.1: Boiling and Condensation You have been studying changes of state for quite a while now. You have studied melting, freezing or solidifying,
The table gives information about some ways of reducing the energy consumption in a house. Installation cost in. Fit a new hot water boiler
 ## (a) The table gives information about some ways of reducing the energy consumption in a house. Method of reducing energy consumption Installation cost in Annual saving on energy bills in Fit a new hot
## (a) The table gives information about some ways of reducing the energy consumption in a house. Method of reducing energy consumption Installation cost in Annual saving on energy bills in Fit a new hot
Heat Transfer. Heat Transfer. Thermal Equilibrium. Thermal Inequilibrium
 Heat Transfer, Radiation Convection and Heat Transfer Heat is a form of energy. Heat travels from higher temperature(hotter) region to lower temperature(cooler) region. Two bodies are in thermal equilibrium
Heat Transfer, Radiation Convection and Heat Transfer Heat is a form of energy. Heat travels from higher temperature(hotter) region to lower temperature(cooler) region. Two bodies are in thermal equilibrium
Science Test: Heat Energy
 Science Test: Heat Energy Name: Date: Section 1: Vocabulary Select the word below that makes the statement correct. Then write the word in the blank. freeze conductor heat friction melt evaporate radiation
Science Test: Heat Energy Name: Date: Section 1: Vocabulary Select the word below that makes the statement correct. Then write the word in the blank. freeze conductor heat friction melt evaporate radiation
Thermal Energy. Conduction, Convection, and Radiation. Before You Read. Read to Learn. Conduction. section 2
 chapter 5 Thermal Energy section 2 Conduction, Convection, and What You ll Learn the three ways heat is transferred the difference between insulators and conductors how insulators control the transfer
chapter 5 Thermal Energy section 2 Conduction, Convection, and What You ll Learn the three ways heat is transferred the difference between insulators and conductors how insulators control the transfer
Test Tube Rack. Test Tube
 Equipment Cards Test Tube A test tube is a tube-shaped piece of glassware or plastic used for holding substances during experiments. Micro test tubes are smaller test tubes that reduce waste and expense.
Equipment Cards Test Tube A test tube is a tube-shaped piece of glassware or plastic used for holding substances during experiments. Micro test tubes are smaller test tubes that reduce waste and expense.
THIRD GRADE SCIENCE (SCIENCE3_4)
 Name: Date: THIRD GRADE SCIENCE (SCIENCE3_4) 1. If you put a metal spoon in hot water, the spoon will A. melt. B. dissolve. C. get cold. D. get hot. 2. When one end of a steel rod is held in a flame, the
Name: Date: THIRD GRADE SCIENCE (SCIENCE3_4) 1. If you put a metal spoon in hot water, the spoon will A. melt. B. dissolve. C. get cold. D. get hot. 2. When one end of a steel rod is held in a flame, the
6. Within an internal combustion engine, the can-shaped component that moves up and down the cylinder
 Student ID: 22133336 Exam: 986052RR - Heat When you have completed your exam and reviewed your answers, click Submit Exam. Answers will not be recorded until you hit Submit Exam. If you need to exit before
Student ID: 22133336 Exam: 986052RR - Heat When you have completed your exam and reviewed your answers, click Submit Exam. Answers will not be recorded until you hit Submit Exam. If you need to exit before
Heat and Specific Heat
 Heat and Specific Heat A person puts a pan on a stove heating ring and returns a few seconds later to find that the pan is hot. The same person puts a pan of water on the stove ring and returns minutes
Heat and Specific Heat A person puts a pan on a stove heating ring and returns a few seconds later to find that the pan is hot. The same person puts a pan of water on the stove ring and returns minutes
Some Demonstration Experiments: Effects of Air Motion, Evaporation, and Pressure Changes on Temperature
 METR 104: Our Dynamic Weather (Lecture w/lab) Some Demonstration Experiments: Effects of Air Motion, Evaporation, and Pressure Changes on Temperature Dr. Dave Dempsey Department of Earth & Climate Sciences
METR 104: Our Dynamic Weather (Lecture w/lab) Some Demonstration Experiments: Effects of Air Motion, Evaporation, and Pressure Changes on Temperature Dr. Dave Dempsey Department of Earth & Climate Sciences
Answer Coming to A Boil Questions
 Answer Coming to A Boil Questions COMING TO A BOIL 1) You are bringing a big pot of cold water to a boil to cook some potatoes. To do it using the least amount of energy you should: a) turn the heat on
Answer Coming to A Boil Questions COMING TO A BOIL 1) You are bringing a big pot of cold water to a boil to cook some potatoes. To do it using the least amount of energy you should: a) turn the heat on
Downloaded from
 Do you know, why? ASSIGNMENTS TUTORIAL HEAT 1. The thick glass tumblers break when hot liquid is poured into them Ans: If boiling hot water is poured in a thick glass tumbler, it cracks because glass is
Do you know, why? ASSIGNMENTS TUTORIAL HEAT 1. The thick glass tumblers break when hot liquid is poured into them Ans: If boiling hot water is poured in a thick glass tumbler, it cracks because glass is
FS 231: Final Exam (5-6-05) Part A (Closed Book): 60 points
 Name: Start time: End time: FS 231: Final Exam (5-6-05) Part A (Closed Book): 60 points 1. What are the units of the following quantities? (10 points) a. Enthalpy of a refrigerant b. Dryness fraction of
Name: Start time: End time: FS 231: Final Exam (5-6-05) Part A (Closed Book): 60 points 1. What are the units of the following quantities? (10 points) a. Enthalpy of a refrigerant b. Dryness fraction of
Determination of the enthalpy of vaporization of liquids
 Related concepts Enthalpy of vaporization, enthalpy of condensation, enthalpy of sublimation, vapour pressure, entropy of vaporization, Clapeyron-Clausius equation, Trouton s rule, laws of thermodynamics,
Related concepts Enthalpy of vaporization, enthalpy of condensation, enthalpy of sublimation, vapour pressure, entropy of vaporization, Clapeyron-Clausius equation, Trouton s rule, laws of thermodynamics,
Use each of the terms in the box to explain how heat is lost from inside a house through the window. conduction convection radiation
 Q1. The diagram shows a side view of a double-glazed window. (a) Use each of the terms in the box to explain how heat is lost from inside a house through the window. conduction convection radiation..................
Q1. The diagram shows a side view of a double-glazed window. (a) Use each of the terms in the box to explain how heat is lost from inside a house through the window. conduction convection radiation..................
Chemistry Materials Separation Processes
 Chemistry Materials Separation Processes You will be assigned to separate a mixture of several different materials. However, first you will need to learn about several techniques used for mixture separation.
Chemistry Materials Separation Processes You will be assigned to separate a mixture of several different materials. However, first you will need to learn about several techniques used for mixture separation.
Freezing (solid), Melting (liquid), Evaporation (gas) Study Presentation INCLUDING WATER STATE CHANGE GRAPHS
 Freezing (solid), Melting (liquid), Evaporation (gas) Study Presentation INCLUDING WATER STATE CHANGE GRAPHS Lesson Vocabulary 1. Melting- solids gain heat and change into liquids 2. Freezing- liquids
Freezing (solid), Melting (liquid), Evaporation (gas) Study Presentation INCLUDING WATER STATE CHANGE GRAPHS Lesson Vocabulary 1. Melting- solids gain heat and change into liquids 2. Freezing- liquids
Week 1 Day 1-2 (combine) Thermal Energy
 Week 1 Day 1-2 (combine) Thermal Energy Heat Transfer Conduction, Convection and Radiation Thermal Energy Transfer Thermal energy transfer is heat moving from a warmer object to a cooler object. This is
Week 1 Day 1-2 (combine) Thermal Energy Heat Transfer Conduction, Convection and Radiation Thermal Energy Transfer Thermal energy transfer is heat moving from a warmer object to a cooler object. This is
1. What are the scales of temperature? What are the formulas to convert among them? Fahrenheit, Celsius, Kelvin
 -* Name Period 1. What are the scales of temperature? What are the formulas to convert among them? Fahrenheit, Celsius, Kelvin 2. Define Heat and temperature. Heat transfer of thermal energy from hotter
-* Name Period 1. What are the scales of temperature? What are the formulas to convert among them? Fahrenheit, Celsius, Kelvin 2. Define Heat and temperature. Heat transfer of thermal energy from hotter
Making a Carousel Lantern. Grade 7 Activity Plan
 Making a Carousel Lantern Grade 7 Activity Plan 1 Reviews and Updates - Carousel Lanterns Activity added by Fola Akpan in July 2017 2 Making a Carousel Lantern Objectives: 1. To compare two methods of
Making a Carousel Lantern Grade 7 Activity Plan 1 Reviews and Updates - Carousel Lanterns Activity added by Fola Akpan in July 2017 2 Making a Carousel Lantern Objectives: 1. To compare two methods of
Heat. Energy. Lesson 2. Unit 2: Water! From Waves to Weather
 Unit 2: Water! From Waves to Weather Heat Energy Lesson 2 Unit 2: Water! From Waves to Weather Heat Energy Lesson 2 Heat energy makes molecules move faster. Students learn to explain heat energy observed
Unit 2: Water! From Waves to Weather Heat Energy Lesson 2 Unit 2: Water! From Waves to Weather Heat Energy Lesson 2 Heat energy makes molecules move faster. Students learn to explain heat energy observed
Keep It Hot! Handout Answer Key
 Keep It Hot! Handout Answer Key Day 1: Introduction Today, you will study heat transfer by conduction and convection! Fill in the rest of these statements: Heat is the transfer or flow of thermal energy.
Keep It Hot! Handout Answer Key Day 1: Introduction Today, you will study heat transfer by conduction and convection! Fill in the rest of these statements: Heat is the transfer or flow of thermal energy.
(1) ... (2) Each strip has a negative charge. The cloth is left with a... charge. This is because particles called... have been transferred
 Q1. A student did an experiment with two strips of polythene. She held the strips together at one end. She rubbed down one strip with a dry cloth. Then she rubbed down the other strip with the dry cloth.
Q1. A student did an experiment with two strips of polythene. She held the strips together at one end. She rubbed down one strip with a dry cloth. Then she rubbed down the other strip with the dry cloth.
Changes of State pg K
 Changes of State pg. 48-53 K Key Terms Melting melting point freezing vaporization Evaporation boiling boiling point condensation Sublimation 48K Picture an ice cream cone on a hot summer day. The ice
Changes of State pg. 48-53 K Key Terms Melting melting point freezing vaporization Evaporation boiling boiling point condensation Sublimation 48K Picture an ice cream cone on a hot summer day. The ice
Parents and Educators: use #CuriousCrew #CuriosityGuide to share what your Curious Crew learned!
 Investigation: 01 The Unpoppable Water Balloon Why won t this balloon pop? 2 balloons Water Basin Safety glasses Candle Matches Adult supervision 1) Put on safety glasses 2) Place the candle in a shallow
Investigation: 01 The Unpoppable Water Balloon Why won t this balloon pop? 2 balloons Water Basin Safety glasses Candle Matches Adult supervision 1) Put on safety glasses 2) Place the candle in a shallow
Well Insulated Houses: Helping to Stay Warm in Winter and Cool in Summer
 CON EDISON WEB-BASED MIDDLE SCHOOL ACTIVITY Well Insulated Houses: Helping to Stay Warm in Winter and Cool in Summer Overview In this activity, you and your students will build two house models from discarded
CON EDISON WEB-BASED MIDDLE SCHOOL ACTIVITY Well Insulated Houses: Helping to Stay Warm in Winter and Cool in Summer Overview In this activity, you and your students will build two house models from discarded
Figure 1. Explain how rubbing an acetate rod with a cloth causes the rod and cloth to become charged
 Q1.A student rubs an acetate rod with a cloth. Figure 1 shows the charges on the acetate rod and cloth before and after rubbing. Figure 1 (a) Explain how rubbing an acetate rod with a cloth causes the
Q1.A student rubs an acetate rod with a cloth. Figure 1 shows the charges on the acetate rod and cloth before and after rubbing. Figure 1 (a) Explain how rubbing an acetate rod with a cloth causes the
HEAT TRANSFER. The tips of both brass rods are held in the gas flame. Mark each of the following as True or False.
 HEAT TRANSFER Refer to the following information for the next four questions. The tips of both brass rods are held in the gas flame. Mark each of the following as or. 1. Heat is conducted only along Rod
HEAT TRANSFER Refer to the following information for the next four questions. The tips of both brass rods are held in the gas flame. Mark each of the following as or. 1. Heat is conducted only along Rod
Heat: Activity: Friction: Temperature: What is Heat? Names. What is heat? How would you define or describe it?
 Names What is Heat? What is heat? How would you define or describe it? Heat: Heat is thermal energy flowing from warmer to cooler objects. Thermal energy is the total energy of the particles of matter.
Names What is Heat? What is heat? How would you define or describe it? Heat: Heat is thermal energy flowing from warmer to cooler objects. Thermal energy is the total energy of the particles of matter.
February 18, What is heat? Touch each image to see how the water molecules react.
 What is heat? Touch each image to see how the water molecules react. Where does heat come from? Touch each image and discuss. How can motion create heat? Touch each image to view the different energy levels
What is heat? Touch each image to see how the water molecules react. Where does heat come from? Touch each image and discuss. How can motion create heat? Touch each image to view the different energy levels
Page 22a. What heats up faster, sand or water? Which one has a greater specific heat capacity?
 Page 22a Have weather map face-up on table Objective: We will describe the three types of heat transfer and explain their roles in Earth processes. Warm-up: What heats up faster, sand or water? Which one
Page 22a Have weather map face-up on table Objective: We will describe the three types of heat transfer and explain their roles in Earth processes. Warm-up: What heats up faster, sand or water? Which one
> Define temperature in terms of the average kinetic energy of atoms or molecules.
 SECTION 1 Temperature KEY TERMS temperature thermometer absolute zero heat temperature a measure of how hot (or cold) something is; specifically, a measure of the average kinetic energy of the particles
SECTION 1 Temperature KEY TERMS temperature thermometer absolute zero heat temperature a measure of how hot (or cold) something is; specifically, a measure of the average kinetic energy of the particles
2018 Year 11 Physics Week 8. Thermal Energy Transfer
 2018 Year 11 Physics Week 8 Thermal Energy Transfer Thermal energy Thermal or heat energy is the energy that flows from a hot region to a cold region by one or more of the processes of: CONDUCTION CONVECTION
2018 Year 11 Physics Week 8 Thermal Energy Transfer Thermal energy Thermal or heat energy is the energy that flows from a hot region to a cold region by one or more of the processes of: CONDUCTION CONVECTION
Solar Matters III Teacher Page
 Solar Matters III Teacher Page We re In Hot Water Now Student Objective The student: will be able to explain how a solar thermal water heating system works will be able to explain conduction, convection
Solar Matters III Teacher Page We re In Hot Water Now Student Objective The student: will be able to explain how a solar thermal water heating system works will be able to explain conduction, convection
LETTER TO FAMILY. Science News. Cut here and paste onto school letterhead before making copies.
 LETTER TO FAMILY Cut here and paste onto school letterhead before making copies. Dear Family, Water is a unique earth material, the only material on Earth that occurs naturally in all three states of matter:
LETTER TO FAMILY Cut here and paste onto school letterhead before making copies. Dear Family, Water is a unique earth material, the only material on Earth that occurs naturally in all three states of matter:
6 th Grade Conduction, Convection, and Stored Heat Energy
 6 th Grade Conduction, Convection, and Stored Heat Energy Summary: Students feel convection by melting an ice cube in their hands. They layer cold water, room temperature water, and hot water in a clear
6 th Grade Conduction, Convection, and Stored Heat Energy Summary: Students feel convection by melting an ice cube in their hands. They layer cold water, room temperature water, and hot water in a clear
Thermal Energy On a sunny day you can feel
 6 Thermal Energy On a sunny day you can feel the energy from the Sun as heat. The dishes shown here collect some of that energy and convert it into other forms of energy. How does heat energy travel through
6 Thermal Energy On a sunny day you can feel the energy from the Sun as heat. The dishes shown here collect some of that energy and convert it into other forms of energy. How does heat energy travel through
Melting and Freezing. STEM Activity 1: What melts in the heat of the sun? Background information and Science information
 1 STEM Activity 1: What melts in the heat of the sun? Background information and Science information All solids have a melting point: the temperature when they change from solid to liquid. Most substances
1 STEM Activity 1: What melts in the heat of the sun? Background information and Science information All solids have a melting point: the temperature when they change from solid to liquid. Most substances
Physical Mechanism of Convection. Conduction and convection are similar in that both mechanisms require the presence of a material medium.
 Convection 1 Physical Mechanism of Convection Conduction and convection are similar in that both mechanisms require the presence of a material medium. But they are different in that convection requires
Convection 1 Physical Mechanism of Convection Conduction and convection are similar in that both mechanisms require the presence of a material medium. But they are different in that convection requires
