Instruction of use. Flexible Naso-Pharyngo- Laryngofiberscope,
|
|
|
- Jeffery McDaniel
- 5 years ago
- Views:
Transcription
1 Page 1 of 9
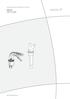
2 Legend 1 Ocular 2 Focussing ring 3 Working channel for flexible instruments 4 Bending protection 5 Deflectable, distal end 6 Lever for control of distal end 7 Fixation thread for pressure compensation cap 8 ACM / British Standard light guide connection 9 Wolf / HSW light guide connection 10 Aesculap / Storz light guide connection 11 pressure compensation cap 12 Cap for leak test 13 Manometer Symbols on product and packaging Warning: General danger signal. Indicates a hazard. If not avoided, the hazard can result in death or serious injury. Warning: refer to accompanying documents. Caution: Indicates a potential hazard. If not avoided, this hazard may result in minor personal injury or product/property damage. Manufacturer Purpose The flexible controllable endoscope is used for visualization of body cavities in the area of ear-nose-throat (ENT). Safe handling and preparation Risk of injury due to defect product! 1. Only use faultless product. The product and accessory may only be used by persons who have the needed training, knowledge and experience. Read, observe and store instructions for use. Only use the product for its intended use, see chapter purpose. Thoroughly clean the new product after removing the transport packaging and before first sterilization (manually or mechanically). Store the new or unused product at a dry, clean and protected place. Visually check product before each use regarding: cleanness, function and damages, e. g. loose, bent, cracked, used up or broken parts. Do not use damaged or defect products. Immediately remove damaged products. Replace damaged parts immediately by original spare parts. Operation Danger of injury and/or malfunction! Do functional check before each use. Risk of burns to patient and user, caused by high-intensity light! The light guide end at the instrument side can reach temperatures, which can cause burns. Make certain the distal end of the endoscope or optical cable connection does not touch human tissue or any Page 2 of 9
3 flammable or heat-sensitive materials while the light source is active. Do not put the endoscope on the patient. Do not touch the distal end of the endoscope and the optical cable connection. Adjust the light source to the minimum required power for optimal illumination of the endoscopic image. Only use light sources of a power rating of up to 180 W. Operation of product The product is equipped with an ocular 1 corresponding to DIN norm. The image can be viewed directly looking into the ocular or on the monitor, using a camera. The focussing ring 2 at the ocular is used to focus the image. The connection for the light guide is suitable for the most popular adapter systems 7. The deflection of the distal end 4 is effected by moving up and down the lever 5 at the main body. The product is delivered with an additional pressure compensation cap 8 and a leak tester 9, 10. The use of those components is explained in chapter Validated reprocessing. Functional and visual inspection Check product regarding damages before each use to exclude endangering of the patient. Carefully carry out the inspection of the product. Due to their small outer diameter they have an increased risk to break. The change over main body/flexible part is particularly at risk. This area is protected by a special bending protection 3. Check the complete coating tube regarding outer damages. Breaks or holes have to be repaired by the manufacturer. Haze of the distal objective glass 11, proximal ocular glass 12 and entrance surface at the light guide connection 7 can usually be cleaned with a pad moistened with alcohol. If those hazes cannot be removed the product has to be sent to the manufacturer to be inspected. The image quality can be checked by focussing an object within the given depth of field. The object has to be clearly visible. Black points show breaks. A bigger quantity of those points means a deterioration of the image quality. In this case a repair by the manufacturer is necessary. Hold the probe with the light guide against a light source and check the distal end. Uniform bright spots signalize an intact illumination 13. Damages and interferences of function due to use of unsuitable or wrong accessory! Other accessory than indicated in the instructions for use may only be used if the characteristics and safety requirements for the intended application will not be influenced. Damages at the deflection due to wrong handling! Only operate the deflection with lever 5. If a resistance can be felt when operating the deflection, do not try to forcibly reach a higher deflection. When pulling out the product of the body the distal end may not be deflected. Damages due to wrong use of the pressure compensation cap! During cleaning, disinfection and wet chemical cold sterilization the pressure compensation cap 8 may not be screwed onto the connection 6. During gas sterilization the pressure compensation cap 8 must be screwed onto the connection 6. Always clean product immediately after use. The distal end is not electrically insulated against the main body. Only use product with light sources which have a spare lamp. If the product is used with electromedical devices, the BF regulations have to be kept. Disassembly Screw off adapter 9, 10. Remove sealing cap compensation cap from Luer Lock connection. Assembly Page 3 of 9
4 Screw on adapter 9, 10. Attach sealing cap to Luer Lock connection. Validated processing procedure Note Adhere to national statutory regulations, international standards and guidelines, and local, clinical hygiene instructions for sterile processing. Note Successful processing of this medical product can only be ensured if processing is peformed through a validated processing procedure. The user/processor is responsible for the validation. General hints To avoid unnecessary, excessive contamination of the complete instrument tray during operations, take care that contaminated instruments are collected separately and not put back into the instrument tray. Encrusted or fixated residues from surgery can make the cleaning process more difficult or ineffective, and can cause corrosion of stainless steel. Therefore the time interval between application and cleaning should not exceed 6 h, no potentially fixating pre-cleaning temperatures > 45 C be applied, and no fixating disinfectants (active ingredient: aldehyde, alcohol) be used. Excessive doses of neutralizers or basic detergents can cause chemical degradation and/or fading and obliteration of laser inscriptions on stainless steel surfaces, regarding visual reading and machinereadability of the inscriptions. Residues containing chlorine or chlorides e. g. in surgical residues, medicines, saline solutions and in the service water used for cleaning, disinfecting and sterilization will cause corrosion damage (pitting, stress corrosion) and result in the destruction of the stainless steel products. To remove such residues, the products must be rinsed sufficiently with fully desalinated water and dried thoroughly. Only use process chemicals that have been tested and approved (e.g. VAH/DGHM or FDA approval or CE mark) and which are compatible with the product s materials according to the chemical manufacturers recommendations. All process parameters specified by the chemical s manufacturer, such as temperatures, concentrations and exposure times, must be strictly observed. Failure to do so can result in the following problems: Optical deterioration, e.g. fading or discoloration of titanium or aluminum surfaces. For aluminum, ph>8 in the application/process solution can already cause visible surface changes. Material damage, e.g. corrosion, cracks, fracturing, premature aging or swelling. Do not use process chemicals that would cause stress cracking or brittleness of plastics. Clean the product immediately after use. Crude pollution can be removed manually with a soft cloth or a soft brush. Following the further common steps of the processing. For further detailed advice on hygienically safe and material-/value-preserving reprocessing, see Use appropriate cleaning/disinfecting agents if the product is put away in wet condition. To prevent foam formation and reduced effectiveness of the process chemicals: Prior to mechanical cleaning and disinfection, rinse the product thoroughly with running water. The bending radius of the flexible endoscope part may not be smaller than 8 cm. Avoid excessive pressure onto the product. Clean used cleaning brushes after each use in ultrasonic bath and disinfect it afterwards. At the end of the day, store the used brushes after cleaning and disinfection dry and protected against contamination. Do not use the product in case of breakage or kinking of the wire. Page 4 of 9
5 Preparations at the place of application Disassemble the product immediately after use, as described in these instructions for use, see Disassembling. Remove the sealing cap from Luer Lock connector. Rinse surfaces that cannot be visually inspected, e.g. on products with hidden crevices or lumens or products with complex geometries, preferably with distilled water, using e.g. a disposable syringe. Remove visible surgical residues as completely as possible, using a lint-free wet wipe. Put the dry product into a closed disposal container and have it transferred to cleaning and disinfection within 6 h. Preparation before cleaning Disassemble the product prior to cleaning, see Disassembling. Carry out leak test. Damages due to reprocessing of untight product/leak test! To avoid damages, carry out leak test before each cleaning and disinfection. Screw pressure compensation cap for leak test 12 onto thread 7 of the product. Increase pressure by operating the balloon pump until the pressure reaches mmhg. After reaching this value, observe display of the manometer. A sudden significant decrease in pressuresignalizesleakage. In this case the product has to be repaired before using it again. To do so, send it dry and in a tight case with the marking not disinfected to the manufacturer. If the pressure will not decrease the product has no untight areas and can be reprocessed according to the cleaning and disinfection schedule. Cleaning/Disinfection Damages to the product due to inappropriate cleaning/disinfection agents and/or excessive high temperatures! Use cleaning and disinfection agents according to the manufacturer s instructions. The cleaning an disinfecting agents must - be approved for flexible endoscopes - not attack softeners (e.g. silicone) Observe specifications regarding concentration, temperature, exposure time and use-by-date. Do not exceed the maximum allowable cleaning temperature of 30 C. Damage to the optical system caused by loosening of connections during ultrasound cleaning! Do not clean the product in ultrasonic bath. Damages at the endoscope due to mechanical cleaning! Do not clean the product mechanically. Manual cleaning/disinfection Clean product with flexible joints in open position, respectively move joints. Inspect visible surfaces for residual contamination after manual cleaning/disinfection. Repeat the cleaning process if necessary. Do not insert the product in cleaning/disinfectant agent over night. Damages at the product due to wrong handling! Only reprocess product if it is tight (see leak test). During reprocessing, the product must be in a stretched position, respectively the bending radius must not be smaller than 8 cm. Avoid excessive pressure or tension on the flexible probe. Do not insert the product in cleaning/disinfectant agent over night. Page 5 of 9
6 Manual cleaning with immersion disinfection Stage Step T ( C/ F) t (min) Conc. [%] Water quality Chemical I Cleaning 34-45/ D-W B. Braun Cleaner N II Intermediate RT (cold) 3 x 1 n.a. D-W n.a. rinse III Disinfection 20-25/ ,5 D-W B. Braun HelipurHplusN IV Final rinse RT (cold) 3 x 2 n.a. FD-W sterile n.a. V Drying RT n.a. n.a. n.a. n.a. D-W: FD-W: RT: Drinking water Fully desalinated water (demineralized, low microbiological contamination: max. 10 microorganisms/ml, low endotoxin: max endotoxin units/ml) Room temperature Stage I Fully immerse the product in the cleaning solution. Make certain that all accessible surfaces are moistened and all lumens, channels and complex geometries are filled with cleaning agent, free of bubbles. Clean the product immersed in the cleaning solution, using a soft, lint free disposable tissue from outside. Brush through all surfaces that are not accessible to visual inspection, e.g. in products with hidden crevices, lumens (e. g. working channel) or complex geometries with the enclosed brush for at least 1 min or until no more residues can be removed. Observe that the brush will be pulled through the working channel. To do so, insert the wire end at the proximal end of the working channel (Luer Lock) and carefully push it forward until the wire can be catched at the distal end. Then pull the brush slowly through the working channel to remove residues from the inner surfaces of the channel. Mobilize non-rigid components, e.g. set screws, joints, slides, etc. 3 times in each direction to the positive stop. After cleaning, thoroughly rinse through these components (at least five times) with the cleaning disinfectant, using a disposable syringe (20 ml). The working channels must be cleaned by mechanical cleaning (at least 1 minute) with the enclosed brush and by rinsing thoroughly (at least 5 times) with the cleaning solution, using a disposable syringe (20 ml). Do not use metal cleaning brushes or other abrasives that would damage the product surfaces and could cause corrosion. Stage II Completely rinse through the product (all accessible surfaces) 3 times for at least 1 minute and use fresh water for every rinse. Mobilize non-rigid components, e.g. set screws, joints, slides, etc. 3 times in each direction to the positive stop. Thoroughly rinse lumens and channels during each rinse at least 5 times, using a disposable syringe (20 ml). Allow water to drip off for a sufficient length of time. Stage III Fully immerse the product in the disinfecting solution. Make certain that all accessible surfaces are moistened and all lumens, channels and complex geometries are filled with disinfecting solution, free of bubbles. Rinse the lumens at the beginning of the residence time at least 5 times, using a disposable syringe (20 ml) and a suitable rinsing adapter. Mobilize non-rigid components, e.g. set screws, joints, etc. during cleaning 3 times in each direction to the positive stop. Page 6 of 9
7 Stage IV Rinse/rinse through the product completely (all accessible surfaces) 3 times for at least 2 minutes and use fresh water for every rinse. Rinse lumens and channels during every rinse with help of a disposable syringe (20 ml) at least 5 times. Mobilize non-rigid components, e.g. set screws, joints, etc. during cleaning 3 times in each direction to the positive stop. Allow water to drip off for a sufficient length of time. Stage V Thoroughly dry the product with a soft, lint free disposable cloth. Thoroughly blow-dry lumens and channels with air, e.g. of a disposable syringe (50 ml). Do not use compressed air! Inspection, maintenance and checks Cool down product to room temperature. After each complete cleaning, disinfection and drying cycle, check that the product is: dry, clean, operational, and free of damage (e. g. broken insulation or corroded, loose, bent, broken, cracked, worn or fractured components). Dry the product if it is wet or moist. Repeat cleaning and disinfecting of products that still show impurities or contamination. Check the product for proper functioning. Immediately sort out damaged or inoperative products and have them sent to the Service, see Technical Service. Assemble the separable product, see Assembling. Check compatibility with associated products. Packaging Appropriately protect products with fine working tips. Sort the product into its appropriate storage device or put it on a suitable tray. Package trays appropriately for the sterilization process (e. g. in sterile containers). The bending radius of the flexible part may not be smaller than 8 cm. Ensue that the packaging provides sufficient protection against recontamination of the product during storage (DIN EN ISO 11607). Sterilization Note The product must be disas sembled before sterilization. Damages at the product due to wrong sterilization method! Do not sterilize the product in steam and hot air. Do not flash sterilize the product. Do wet chemical cold sterilization only as alternative method if possibilities for different sterilization methods are not given. Damages at the product due to sterilization without pressure compensation cap! For gas sterilization, always screw on the pressure compensation cap onto the light guide connection. Make certain that all external and internal surfaces will be exposed to the sterilizing agent (e. g. by opening and closing the valves and faucets). The bending radius of the flexible part may not be smaller than 8 cm. The sterilization of the product is preferably done by Ethylenoxide sterilization agents according to Sterivit process. Validated sterilization method: ETO - Disassemble product. - Screw on pressure equilibration cap 11 onto the optical waveguide connection 7. - Sterilization according to Sterivit method: Page 7 of 9
8 Gas compound: Sterilization temperature: Chamber pressure: Sterilization time: Degassing time: 6% Ethylene oxide, 94% CO2 55 C±2 C / 131 F±5 F 1.7 bar (170 kpa) 120 minutes minimum 12 hours Storing Store sterile products in a germ-proof packaging under dust protection in a dry, dark and temperature-controlled room. Store the product hanging. If this should not be possible, store product in elongated position. The bending radius of the flexible part may not be smaller than 8 cm. The transport packaging may not be used for storing, due to the risk of recontamination. Maintenance The product is maintenance-free. If the product may get damaged, send it, in the original packaging to avoid transport damages, to the below mentioned service address. Repairs may only be done by persons, which have been authorized to do so by the manufacturer. Only by observing this, guarantee claims will persist. The manufacturer is only responsible for impact to security, reliability and product performance, if the product is used in accordance with the instructions for use. Technical service Risk of injury and/or malfunction! Do not modify the product. For service and repairs, please contact the below mentioned service address. Modifications carried out on medical technical equipment may result in loss of guarantee/warranty rights and forfeiture of applicable licenses. For the protection of your staff, as well as the staff, thoroughly clean and sterilize the product (and, if applicable, accessories) before shipment. If this should, due to pressing reasons, not be possible, process the product as good as possible and mark it accordingly. The repair center can refuse the repair of polluted or decontaminated products due to safety reasons. Service address AS Medizintechnik GmbH Sattlerstrasse Tuttlingen / Germany Technical data Diameter distal end: 4.3 mm Diameter working channel: 1.4 mm Diameter flexible part: 400 mm Max. deflection, up/down: 140 /140 Field of view: 85 Direction of view: 0 Depth of field: 3 mm - Page 8 of 9
9 Disposal Always observe national regulations when disposing of or recycling the product or its components! AS MEDIZINTECHNIK GMBH DOES NOT ACCEPT RESPONSIBILITY IF THIS CUSTOMER INFORMATION HAS BEEN VIOLATED PROVABLY. Page 9 of 9
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Orthopedics USA Instructions for Use/Technical Description Page 1 of 14 Symbols on product packages Caution, general warning symbol Caution, see documentation
Aesculap Implant Systems Aesculap Implant Systems Orthopedics USA Instructions for Use/Technical Description Page 1 of 14 Symbols on product packages Caution, general warning symbol Caution, see documentation
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Orthopedics USA Instructions for Use/Technical Description Page 1 of 16 Symbols on product packages! Caution, general warning symbol Caution, see documentation
Aesculap Implant Systems Aesculap Implant Systems Orthopedics USA Instructions for Use/Technical Description Page 1 of 16 Symbols on product packages! Caution, general warning symbol Caution, see documentation
I.F.U Re/Processing Reusable Medical Devices
 I.F.U Re/Processing Reusable Medical Devices Distributed by: MANUFACTURER: GA 26-09-001 -EN /20130719 Contents 1 Overview of preparation methods... 3 2 Safety and responsibility... 4 3 Explanation of symbols...
I.F.U Re/Processing Reusable Medical Devices Distributed by: MANUFACTURER: GA 26-09-001 -EN /20130719 Contents 1 Overview of preparation methods... 3 2 Safety and responsibility... 4 3 Explanation of symbols...
AS Medizintechnik GmbH Sattlerstrasse 15, Tuttlingen, Germany Tel 07461/ Fax 07461/
 s 65-201-00-65-202-00 01 Stopcock left 02 Spring Cap 03 Cannula CLEANING / STERILIZATION Immediately after use the instruments have to be soaked in a combined disinfection and cleaning solution in order
s 65-201-00-65-202-00 01 Stopcock left 02 Spring Cap 03 Cannula CLEANING / STERILIZATION Immediately after use the instruments have to be soaked in a combined disinfection and cleaning solution in order
Accessories. Maybachstraße. Symmetry Fax: Revised 10/15
 Recommended Care, Cleaning and Sterilization Instructions for Reusable Instruments & Accessories Symmetry Surgical Inc. 3034 Owen Drive, Antioch, TN 37013 USA 1-800-251-3000 Fax: 1-615-964-5566 www.symmetrysurgical.com
Recommended Care, Cleaning and Sterilization Instructions for Reusable Instruments & Accessories Symmetry Surgical Inc. 3034 Owen Drive, Antioch, TN 37013 USA 1-800-251-3000 Fax: 1-615-964-5566 www.symmetrysurgical.com
Processing (cleaning, disinfection and sterilization) of instruments from Smith & Nephew Orthopaedics AG
 Guide Processing (cleaning, disinfection and sterilization) of instruments from Smith & Nephew Orthopaedics AG Acceptance Cleaning, Disinfection Inspection, Packing Use T Sterilization t Storage Return
Guide Processing (cleaning, disinfection and sterilization) of instruments from Smith & Nephew Orthopaedics AG Acceptance Cleaning, Disinfection Inspection, Packing Use T Sterilization t Storage Return
INSTRUCTIONS FOR USE & STERILIZATION FOR INSTRUMENTS
 INSTRUCTIONS FOR USE & STERILIZATION PMS PRECISION MEDICAL SPECIALITIES GMBH Kreuzstrasse 5 DE-78532 Tuttlingen PO Box 4607 DE-78511 Tuttlingen Fon: +49 74 61 13 131 Fax: +49 74 61 76 698 info@pms-tuttlingen.de
INSTRUCTIONS FOR USE & STERILIZATION PMS PRECISION MEDICAL SPECIALITIES GMBH Kreuzstrasse 5 DE-78532 Tuttlingen PO Box 4607 DE-78511 Tuttlingen Fon: +49 74 61 13 131 Fax: +49 74 61 76 698 info@pms-tuttlingen.de
CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization
 CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization 1. WARNINGS AND PRECAUTIONS 1.1. Caution should be exercised when handling instruments with sharp points, or cutting/drilling
CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization 1. WARNINGS AND PRECAUTIONS 1.1. Caution should be exercised when handling instruments with sharp points, or cutting/drilling
CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization
 CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization 1. WARNINGS AND PRECAUTIONS 1.1. Caution should be exercised when handling instruments with sharp points, or cutting/drilling
CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization 1. WARNINGS AND PRECAUTIONS 1.1. Caution should be exercised when handling instruments with sharp points, or cutting/drilling
AS Medizintechnik GmbH Sattlerstrasse 15, Tuttlingen, Germany Tel 07461/ Fax 07461/
 Arthroscopy irrigation cannula Arthroscopy irrigation cannulas 65-201-00-65-202-00 01 Stopcock left 02 Spring Cap 03 Cannula CLEANING / STERILIZATION Immediately after use the instruments have to be soaked
Arthroscopy irrigation cannula Arthroscopy irrigation cannulas 65-201-00-65-202-00 01 Stopcock left 02 Spring Cap 03 Cannula CLEANING / STERILIZATION Immediately after use the instruments have to be soaked
AS Medizintechnik GmbH Sattlerstrasse 15, Tuttlingen, Germany Tel 07461/ Fax 07461/
 General Information Use The Air drill System is a pneumatic powered system used for many applications orthopedic and trauma surgery. To ensure proper operation of the air drill, use only original attachment
General Information Use The Air drill System is a pneumatic powered system used for many applications orthopedic and trauma surgery. To ensure proper operation of the air drill, use only original attachment
Flexible Nasopharyngoscope. Operator Manual. Manufactured by Henke-Sass, Wolf Distributed by Anthony Products, Inc.
 Flexible Nasopharyngoscope Operator Manual Manufactured by Henke-Sass, Wolf Distributed by Anthony Products, Inc. 1. Introduction The flexible nasopharyngoscope is sold in unsterile condition as a reusable
Flexible Nasopharyngoscope Operator Manual Manufactured by Henke-Sass, Wolf Distributed by Anthony Products, Inc. 1. Introduction The flexible nasopharyngoscope is sold in unsterile condition as a reusable
AS Medizintechnik GmbH Sattlerstrasse 15, Tuttlingen, Germany Tel 07461/ Fax 07461/
 HIGH-FLOW Arthroscopy Sheath with one or two rotating Stopcocks HIGH-FLOW Arthroscopy Sheath with one or two rotating Stopcocks 65-227-01-65-240-12 1 Safety and responsibility Before using the instrument
HIGH-FLOW Arthroscopy Sheath with one or two rotating Stopcocks HIGH-FLOW Arthroscopy Sheath with one or two rotating Stopcocks 65-227-01-65-240-12 1 Safety and responsibility Before using the instrument
EMAX 2 PLUS AND XMAX CLEANING AND STERILIZATION BASKET. Mechanical/Automated Cleaning and Sterilization Guide in a Variety of Applications
 EMAX 2 PLUS AND XMAX CLEANING AND STERILIZATION BASKET Mechanical/Automated Cleaning and Sterilization Guide in a Variety of Applications TABLE OF CONTENTS MANUAL PRE-CLEANING High Speed Handpieces 2
EMAX 2 PLUS AND XMAX CLEANING AND STERILIZATION BASKET Mechanical/Automated Cleaning and Sterilization Guide in a Variety of Applications TABLE OF CONTENTS MANUAL PRE-CLEANING High Speed Handpieces 2
Reusable Laryngoscope Blades and Handles
 Manufacturer: Starkling Medizintechnik Method: Re-processing instructions Symbol: N.A. Device(s): 1 Green System Reusable Laryngoscope Blade with integrated Light Guide, Solid Stainless Steel Construction
Manufacturer: Starkling Medizintechnik Method: Re-processing instructions Symbol: N.A. Device(s): 1 Green System Reusable Laryngoscope Blade with integrated Light Guide, Solid Stainless Steel Construction
Instructions for Use Spinal Punches IFU
 Indications for use Spinal punches are manually operated instruments indicated for cutting or biting bone during surgery involving the skull or spinal column. Contraindiciations Instruments should not
Indications for use Spinal punches are manually operated instruments indicated for cutting or biting bone during surgery involving the skull or spinal column. Contraindiciations Instruments should not
CONVENTUS CAGE TM - DR Procedure Instruments Instrument Tray. Equipment Cleaning and Sterilization
 CONVENTUS CAGE TM - DR Procedure Instruments Instrument Tray Equipment Cleaning and Sterilization Table of Contents 1. WARNINGS AND PRECAUTIONS... 3 2. INSTRUMENT PREPARATION... 3 3. CAVITY PREPARATION
CONVENTUS CAGE TM - DR Procedure Instruments Instrument Tray Equipment Cleaning and Sterilization Table of Contents 1. WARNINGS AND PRECAUTIONS... 3 2. INSTRUMENT PREPARATION... 3 3. CAVITY PREPARATION
CLEANING INSTRUCTIONS
 Reusable Instrument Care Manual Pagoda Pedicle Screw System Manufacturer Ortho Development Corporation 12187 South Business Park Drive Draper, UT 84020 Phone 801-553-9991 Fax 801-553-9993 www.orthodevelopment.com
Reusable Instrument Care Manual Pagoda Pedicle Screw System Manufacturer Ortho Development Corporation 12187 South Business Park Drive Draper, UT 84020 Phone 801-553-9991 Fax 801-553-9993 www.orthodevelopment.com
AS Medizintechnik GmbH Sattlerstraße Tuttlingen Germany
 Important information You receive a high-quality product by purchasing this sterile container system. lts proper handling and use will be described in the following. Please read this user manual thoroughly
Important information You receive a high-quality product by purchasing this sterile container system. lts proper handling and use will be described in the following. Please read this user manual thoroughly
2015 Myco Industries, Inc Fax:
 Endoscope Cleaning and Handling It is highly important that you manually clean the endoscope immediately after it is removed from the patient, BEFORE it is automated or manually disinfected. Cleaning your
Endoscope Cleaning and Handling It is highly important that you manually clean the endoscope immediately after it is removed from the patient, BEFORE it is automated or manually disinfected. Cleaning your
Instructions for Use: Fiberscope and LED Light Source
 Instructions for Use: and Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Range of Applications for Product Key Specifications of Product, FBR-001, FBR-002, FBR-ULB-100,
Instructions for Use: and Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Range of Applications for Product Key Specifications of Product, FBR-001, FBR-002, FBR-ULB-100,
Processing Instructions. Cleaning so easy.
 EN Processing Instructions. Cleaning so easy. 2 A good decision. Congratulations on your new tiologic easyclean Wash-Tray. Machine treatment of the entire instrument set is an essential component of quality
EN Processing Instructions. Cleaning so easy. 2 A good decision. Congratulations on your new tiologic easyclean Wash-Tray. Machine treatment of the entire instrument set is an essential component of quality
INSTRUCTIONS FOR CLEANING AND STERILIZATION OF ALL B.BRAUN AESCULAP HAND HELD SURGICAL INSTRUMENTS AND POWER PRODUCTS (UNITED KINGDOM REQUIREMENTS)
 INSTRUCTIONS FOR CLEANING AND STERILIZATION OF ALL B.BRAUN AESCULAP HAND HELD SURGICAL INSTRUMENTS AND POWER PRODUCTS (UNITED KINGDOM REQUIREMENTS) 1 INTRODUCTION This paper is intended to give general
INSTRUCTIONS FOR CLEANING AND STERILIZATION OF ALL B.BRAUN AESCULAP HAND HELD SURGICAL INSTRUMENTS AND POWER PRODUCTS (UNITED KINGDOM REQUIREMENTS) 1 INTRODUCTION This paper is intended to give general
Integra Salto Talaris and Integra XT Non-Sterile Device Reprocessing. Instructions for Use. 4. Manual Cleaning Instructions
 Integra Salto Talaris and Integra XT Non-Sterile Device Reprocessing Instructions for Use. Description and Intended Use Integra LifeSciences Corporation reusable surgical device sets consist of various
Integra Salto Talaris and Integra XT Non-Sterile Device Reprocessing Instructions for Use. Description and Intended Use Integra LifeSciences Corporation reusable surgical device sets consist of various
Operating / User Manual
 UniARM Surgical Support System Operating / User Manual Version 1.1 MITAKA KOHKI Co., Ltd. 1. Introduction Introduction Thank you for purchasing the UniARM for use in surgical procedures. To use the device
UniARM Surgical Support System Operating / User Manual Version 1.1 MITAKA KOHKI Co., Ltd. 1. Introduction Introduction Thank you for purchasing the UniARM for use in surgical procedures. To use the device
Instructions for use. BA Optima. Air motors BA602 (BA640081) BA604 (BA640060)
 Instructions for use BA Optima Air motors BA602 (BA640081) BA604 (BA640060) Contents 1. Introduction....3 5 2. Safety notes...6 9 3. Product description... 10 11 4. Operation... 12 13 Assembly/Removal,
Instructions for use BA Optima Air motors BA602 (BA640081) BA604 (BA640060) Contents 1. Introduction....3 5 2. Safety notes...6 9 3. Product description... 10 11 4. Operation... 12 13 Assembly/Removal,
Table of Contents. English
 OM-E0799E 000 English Thank you for purchasing VIVA ace Motor Kit. Please read this Operation Manual and the VIVA ace Basic Set Operation Manual carefully before use for operating instructions and care
OM-E0799E 000 English Thank you for purchasing VIVA ace Motor Kit. Please read this Operation Manual and the VIVA ace Basic Set Operation Manual carefully before use for operating instructions and care
Cleaning and Sterilisation for Burs, accessories and filling material
 Cleaning and Sterilisation for Burs, accessories and filling material EN FOR DENTAL USE ONLY CLEANING AND STERILISATION PROCEDURE FOR BURS, ACCESSORIES AND FILLING MATERIAL 1) FOREWORD Devices that are
Cleaning and Sterilisation for Burs, accessories and filling material EN FOR DENTAL USE ONLY CLEANING AND STERILISATION PROCEDURE FOR BURS, ACCESSORIES AND FILLING MATERIAL 1) FOREWORD Devices that are
Cleaning and Sterilization for Instruments & Posts
 Cleaning and Sterilization for Instruments & Posts EN FOR DENTAL USE ONLY CLEANING AND STERILIZATION PROCEDURE FOR ENDODONTIC FILES, HAND INSTRUMENTS, PINS AND POSTS, STAINLESS STEEL DRILLS, STAINLESS
Cleaning and Sterilization for Instruments & Posts EN FOR DENTAL USE ONLY CLEANING AND STERILIZATION PROCEDURE FOR ENDODONTIC FILES, HAND INSTRUMENTS, PINS AND POSTS, STAINLESS STEEL DRILLS, STAINLESS
OPERATION MANUAL. (Cleaning, Disinfection and Storage) Endoscope ED-530XT
 US Endoscope ED-530XT OPERATION MANUAL (Cleaning, Disinfection and Storage) Thank you for purchasing our product. Read this manual carefully before use to avoid unexpected accidents and to take full advantage
US Endoscope ED-530XT OPERATION MANUAL (Cleaning, Disinfection and Storage) Thank you for purchasing our product. Read this manual carefully before use to avoid unexpected accidents and to take full advantage
STERITITE INSTRUCTIONS FOR USE
 STERITITE INSTRUCTIONS FOR USE If a rigid container system is used, the manufacturers instructions regarding set preparation and assembly should be followed. -ANSI/AAMI ST79: 2010 & A1:2010 Notes from
STERITITE INSTRUCTIONS FOR USE If a rigid container system is used, the manufacturers instructions regarding set preparation and assembly should be followed. -ANSI/AAMI ST79: 2010 & A1:2010 Notes from
Caution: Federal U.S. law restricts this device to sale by or on the order of a physician.
 Reprocessing Manual Fuse 1C Colonoscope Part Number FSE-056-EN-2.00 Released August, 2013. This manual includes complete reprocessing instructions for the Fuse 1C Colonoscope system. Please refer to the
Reprocessing Manual Fuse 1C Colonoscope Part Number FSE-056-EN-2.00 Released August, 2013. This manual includes complete reprocessing instructions for the Fuse 1C Colonoscope system. Please refer to the
IMPORTANT INFORMATION ON THE DEPUY SYNTHES
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE DEPUY SYNTHES MatrixNEURO RECONSTRUCTION MESH, STERILE MatrixNEURO PREFORMED MESH, STERILE GP2949-B-CAN DESCRIPTION The DePuy Synthes
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE DEPUY SYNTHES MatrixNEURO RECONSTRUCTION MESH, STERILE MatrixNEURO PREFORMED MESH, STERILE GP2949-B-CAN DESCRIPTION The DePuy Synthes
SterilContainer S for use in the Steris V-PRO 60 Low Temperature Sterilization System
 SterilContainer S for use in the Steris V-PRO 60 Low Temperature Sterilization System This document contains Instructions for Use regarding the processing of the SterilContainer S prior to sterilization
SterilContainer S for use in the Steris V-PRO 60 Low Temperature Sterilization System This document contains Instructions for Use regarding the processing of the SterilContainer S prior to sterilization
Cleaning & Sterilization
 Handpieces & Attachments Instruments Cleaning & Sterilization System 7 System 6 System 5 CD4 CD3 SABO 2 SABO RemB CORE TPS Processing Instructions User/Patient Safety all contribute to the efficacy of
Handpieces & Attachments Instruments Cleaning & Sterilization System 7 System 6 System 5 CD4 CD3 SABO 2 SABO RemB CORE TPS Processing Instructions User/Patient Safety all contribute to the efficacy of
Processing instructions for the Ligamys suture forceps
 Processing instructions for the Ligamys suture forceps Table of contents 1 Scope of application 4 2 Warnings and precautions 4 5 3 Restrictions 6 4 Processing instructions 7 4.1 Processing immediately
Processing instructions for the Ligamys suture forceps Table of contents 1 Scope of application 4 2 Warnings and precautions 4 5 3 Restrictions 6 4 Processing instructions 7 4.1 Processing immediately
CLEANING AND HANDLING OF WRIGHT INSTRUMENTS
 CLEANING AND HANDLING OF WRIGHT INSTRUMENTS 150824-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
CLEANING AND HANDLING OF WRIGHT INSTRUMENTS 150824-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
RECOMMENDATIONS FOR CLEANING DEVICES
 RECOMMENDATIONS FOR CLEANING D E C O N TA M I N AT I O N A N D S T E R I L I Z AT I O N O F M E D A C T A I N T E R N A T I O N A L O R T H O P E D I C DEVICES 1 This document was prepared to provide cleaning,
RECOMMENDATIONS FOR CLEANING D E C O N TA M I N AT I O N A N D S T E R I L I Z AT I O N O F M E D A C T A I N T E R N A T I O N A L O R T H O P E D I C DEVICES 1 This document was prepared to provide cleaning,
V. Mueller. V. Mueller Reusable Devices: Cleaning and Sterilization Guide USA. Rx Only
 V. Mueller en V. Mueller Reusable Devices: Cleaning and Sterilization Guide USA Rx Only V. Mueller, CareFusion and the CareFusion logo are trademarks or registered trademarks of CareFusion Corporation
V. Mueller en V. Mueller Reusable Devices: Cleaning and Sterilization Guide USA Rx Only V. Mueller, CareFusion and the CareFusion logo are trademarks or registered trademarks of CareFusion Corporation
INSTRUCTIONS EVIS EXERA GASTROINTESTINAL VIDEOSCOPE
 INSTRUCTIONS EVIS EXERA GASTROINTESTINAL VIDEOSCOPE OLYMPUS GIF TYPE XP160 OLYMPUS GIF TYPE 160 OLYMPUS GIF TYPE Q160 OLYMPUS GIF TYPE Q160Z OLYMPUS GIF TYPE 1TQ160 OLYMPUS GIF TYPE XTQ160 EVIS EXERA COLONOVIDEOSCOPE
INSTRUCTIONS EVIS EXERA GASTROINTESTINAL VIDEOSCOPE OLYMPUS GIF TYPE XP160 OLYMPUS GIF TYPE 160 OLYMPUS GIF TYPE Q160 OLYMPUS GIF TYPE Q160Z OLYMPUS GIF TYPE 1TQ160 OLYMPUS GIF TYPE XTQ160 EVIS EXERA COLONOVIDEOSCOPE
INSTRUCTIONS EVIS EXERA II DUODENOVIDEOSCOPE
 Single use soft brush (MAJ-1888) INSTRUCTIONS EVIS EXERA II DUODENOVIDEOSCOPE OLYMPUS TJF TYPE Q180V Accessories: Water resistant cap (MH-553) Biopsy valve (MB-358) Air/water valve (MH-438) Suction cleaning
Single use soft brush (MAJ-1888) INSTRUCTIONS EVIS EXERA II DUODENOVIDEOSCOPE OLYMPUS TJF TYPE Q180V Accessories: Water resistant cap (MH-553) Biopsy valve (MB-358) Air/water valve (MH-438) Suction cleaning
FLASH PAK STERILIZATION CONTAINER SYSTEM
 INSTRUCTIONS FOR USE FLASH PAK STERILIZATION CONTAINER SYSTEM Description The Flash Pak Sterilization Container System consists of a family of rigid reusable containers that provide an effective sterilization
INSTRUCTIONS FOR USE FLASH PAK STERILIZATION CONTAINER SYSTEM Description The Flash Pak Sterilization Container System consists of a family of rigid reusable containers that provide an effective sterilization
When you sterilize an instrument set, you know what the standards are.
 Sterilization & Infection Control The importance of cleaning in earnest A regular column on sterilization and infection control issues. When you sterilize an instrument set, you know what the standards
Sterilization & Infection Control The importance of cleaning in earnest A regular column on sterilization and infection control issues. When you sterilize an instrument set, you know what the standards
User Guide for Reprocessing of the SUPER VIEW Wide Angle Viewing System Sterilizable Components and Accessories
 User Guide for Reprocessing of the SUPER VIEW Wide Angle Viewing System Sterilizable Components and Accessories Table of Contents 1. Intended Use Statement... 3 2. Proper Use... 3 3. General Information...
User Guide for Reprocessing of the SUPER VIEW Wide Angle Viewing System Sterilizable Components and Accessories Table of Contents 1. Intended Use Statement... 3 2. Proper Use... 3 3. General Information...
SKLAR COATED SURGICAL INSTRUMENTS RECOMMENDED CARE & CLEANING INSTRUCTIONS
 SKLAR COATED SURGICAL INSTRUMENTS RECOMMENDED CARE & CLEANING INSTRUCTIONS CAUTION: The following instructions are for all Sklar Coated stainless steel instruments. Read instructions prior to use. Improper
SKLAR COATED SURGICAL INSTRUMENTS RECOMMENDED CARE & CLEANING INSTRUCTIONS CAUTION: The following instructions are for all Sklar Coated stainless steel instruments. Read instructions prior to use. Improper
INSTRUCTIONS FOR CLEANING AND STERILISING HUMECA MEDICAL EQUIPMENT FOR BOTH ELECTRIC AND MANUAL DEVICES
 INSTRUCTIONS FOR CLEANING AND STERILISING HUMECA MEDICAL EQUIPMENT FOR BOTH ELECTRIC AND MANUAL DEVICES Contents: Pagina 1 Introduction...2 2 Treatment instructions...2 3 Cleaning...2 3.1 Place of use...4
INSTRUCTIONS FOR CLEANING AND STERILISING HUMECA MEDICAL EQUIPMENT FOR BOTH ELECTRIC AND MANUAL DEVICES Contents: Pagina 1 Introduction...2 2 Treatment instructions...2 3 Cleaning...2 3.1 Place of use...4
ON TRACK Reprocessing In-service / Competency for CF/PCF/GIF Endoscopes (EVIS, EXERA, EXERA II)
 THIS CHECKLIST IS DESIGNED FOR USE SOLELY AS A CUSTOMER EDUCATIONAL TOOL, AND IS NOT INTENDED TO REPLACE OR ANY WAY MODIFY THE OLYMPUS INSTRUCTION MANUAL/REPROCESSING MANUAL. BE SURE TO FOLLOW THE DETAILED
THIS CHECKLIST IS DESIGNED FOR USE SOLELY AS A CUSTOMER EDUCATIONAL TOOL, AND IS NOT INTENDED TO REPLACE OR ANY WAY MODIFY THE OLYMPUS INSTRUCTION MANUAL/REPROCESSING MANUAL. BE SURE TO FOLLOW THE DETAILED
Made in USA. Sunoptic Technologies 6018 Bowdendale Avenue Jacksonville, FL 32216, USA (904)
 Fiber Optic Cables Instructions for Use and Processing Instructions DFU-950-0031-00 Fiber Optic Cables Made in USA Sunoptic Technologies 6018 Bowdendale Avenue Jacksonville, FL 32216, USA (904) 737 7611
Fiber Optic Cables Instructions for Use and Processing Instructions DFU-950-0031-00 Fiber Optic Cables Made in USA Sunoptic Technologies 6018 Bowdendale Avenue Jacksonville, FL 32216, USA (904) 737 7611
Best Practice Guidelines For Cleaning, Disinfection and Sterilization of Critical and Semi-critical Medical Devices
 Best Practice Guidelines For Cleaning, Disinfection and Sterilization of Critical and Semi-critical Medical Devices In BC Health Authorities THIS DOCUMENT IS INTENDED TO DESCRIBE BEST PRACTICES HEALTH
Best Practice Guidelines For Cleaning, Disinfection and Sterilization of Critical and Semi-critical Medical Devices In BC Health Authorities THIS DOCUMENT IS INTENDED TO DESCRIBE BEST PRACTICES HEALTH
INSTRUCTIONS EVIS EXERA II DUODENOVIDEOSCOPE
 Single use soft brush (MAJ-1888) INSTRUCTIONS EVIS EXERA II DUODENOVIDEOSCOPE OLYMPUS TJF TYPE Q180V Accessories: Water resistant cap (MH-553) Biopsy valve (MB-358) Air/water valve (MH-438) Suction cleaning
Single use soft brush (MAJ-1888) INSTRUCTIONS EVIS EXERA II DUODENOVIDEOSCOPE OLYMPUS TJF TYPE Q180V Accessories: Water resistant cap (MH-553) Biopsy valve (MB-358) Air/water valve (MH-438) Suction cleaning
Rigid Reusable & Single use Sigmoidoscopes, Anoscopes, and Accessories ENGLISH 1. Perform reprocessing immediately after each use.
 Rigid Reusable & Single use Sigmoidoscopes, Anoscopes, and Accessories ENGLISH 1 ENGLISH Intended Use The Welch Allyn Rigid Reusable & Single Use Sigmoidoscopes and Anoscopes are used to illuminate and
Rigid Reusable & Single use Sigmoidoscopes, Anoscopes, and Accessories ENGLISH 1 ENGLISH Intended Use The Welch Allyn Rigid Reusable & Single Use Sigmoidoscopes and Anoscopes are used to illuminate and
THE ULTRASONIC DISRUPTION OF ORGANIC SOIL ON COMPLEX INSTRUMENTATION
 CFSA CSSD Conference Durban ICC Wednesday 8 th March 2017 Using technology to improve patient safety THE ULTRASONIC DISRUPTION OF ORGANIC SOIL ON COMPLEX INSTRUMENTATION Helen Loudon Independent Infection
CFSA CSSD Conference Durban ICC Wednesday 8 th March 2017 Using technology to improve patient safety THE ULTRASONIC DISRUPTION OF ORGANIC SOIL ON COMPLEX INSTRUMENTATION Helen Loudon Independent Infection
Operator s Manual. Video Laryngoscope. ( Instructions for use ) Doc no: Revision 1.1 8th January 2013
 Video Laryngoscope Operator s Manual ( Instructions for use ) Doc no: 100-160-000 Revision 1.1 8th January 2013 Aircraft Medical Ltd 2012 www.aircraftmedical.com Contents 1 Introduction 2 1.1 Description
Video Laryngoscope Operator s Manual ( Instructions for use ) Doc no: 100-160-000 Revision 1.1 8th January 2013 Aircraft Medical Ltd 2012 www.aircraftmedical.com Contents 1 Introduction 2 1.1 Description
X99 Series. Dental Handpiece. Instruction For Use
 X99 Series Dental Handpiece Instruction For Use 0413 Table of Contents Symbols... 2 Introduction... 3-4 Before Use... 5 Product description... 6 Technical Specifications... 7 Operation... 8-11 Hygienic
X99 Series Dental Handpiece Instruction For Use 0413 Table of Contents Symbols... 2 Introduction... 3-4 Before Use... 5 Product description... 6 Technical Specifications... 7 Operation... 8-11 Hygienic
PERFORM Operating Document
 PERFORM Operating Document Use and Maintenance of Tuttnauer Table-Top Autoclave PC-POD-CA-006-v03 Revision History Version Reason for Revision Date 01 New POD 13-Aug-13 02 POD Section 3.1.3 and 3.2 revised
PERFORM Operating Document Use and Maintenance of Tuttnauer Table-Top Autoclave PC-POD-CA-006-v03 Revision History Version Reason for Revision Date 01 New POD 13-Aug-13 02 POD Section 3.1.3 and 3.2 revised
Manual Pre-disinfection/Cleaning and Sterilization Instructions for SATELEC Tips and Files
 Manual Pre-disinfection/Cleaning and Sterilization Instructions for SATELEC Tips and Files Cautions Do not use steel wool or abrasive cleaners. Avoid solutions containing iodine or with a high chlorine
Manual Pre-disinfection/Cleaning and Sterilization Instructions for SATELEC Tips and Files Cautions Do not use steel wool or abrasive cleaners. Avoid solutions containing iodine or with a high chlorine
RAP-hip Hip stem extraction set
 Cleaning, sterilisation and maintenance RAP-hip Hip stem extraction set C Distributor: Marketed by: SAFRIMA AG Unterworbenstrasse 51 CH-3252 Worben Switzerland Phone: +41 32 387 05 91 Fax: +41 32 387 05
Cleaning, sterilisation and maintenance RAP-hip Hip stem extraction set C Distributor: Marketed by: SAFRIMA AG Unterworbenstrasse 51 CH-3252 Worben Switzerland Phone: +41 32 387 05 91 Fax: +41 32 387 05
Operator s Guide. Autoclavable Internal Handles with Integrated Paddles Rev. M
 Operator s Guide Autoclavable Internal Handles with Integrated Paddles 9650-0550 Rev. M The issue date for the Autoclavable Internal Handles with Integrated Paddles Operator s Guide (REF 9650-0550 Rev.
Operator s Guide Autoclavable Internal Handles with Integrated Paddles 9650-0550 Rev. M The issue date for the Autoclavable Internal Handles with Integrated Paddles Operator s Guide (REF 9650-0550 Rev.
Manual Pre-disinfection/Cleaning and Sterilization Instructions for SATELEC Tips and Files
 Manual Pre-disinfection/Cleaning and Sterilization Instructions for SATELEC Tips and Files Cautions Do not use steel wool or abrasive cleaners. Avoid solutions containing iodine or with a high chlorine
Manual Pre-disinfection/Cleaning and Sterilization Instructions for SATELEC Tips and Files Cautions Do not use steel wool or abrasive cleaners. Avoid solutions containing iodine or with a high chlorine
Recommendations for cleaning, decontamination and sterilisation. devices
 Recommendations for cleaning, decontamination and sterilisation of MEDACTa INTERNATIONAL orthopedic devices 1 This document was prepared to provide cleaning, decontaminating and sterilising instructions
Recommendations for cleaning, decontamination and sterilisation of MEDACTa INTERNATIONAL orthopedic devices 1 This document was prepared to provide cleaning, decontaminating and sterilising instructions
THE BASICS OF DECONTAMINATION
 THE BASICS OF DECONTAMINATION Objectives Review the basic factors that impact cleaning and decontamination of surgical devices Present the fundamentals of manual and automatic cleaning Review various cleaning
THE BASICS OF DECONTAMINATION Objectives Review the basic factors that impact cleaning and decontamination of surgical devices Present the fundamentals of manual and automatic cleaning Review various cleaning
Classification of Instruments -
 Classification of Instruments - Elaborating of a Reprocessing Instruction According to ISO 17664 Klaus Roth Operation, which have been cancelled due to not sufficient reprocessed instruments Year 2002
Classification of Instruments - Elaborating of a Reprocessing Instruction According to ISO 17664 Klaus Roth Operation, which have been cancelled due to not sufficient reprocessed instruments Year 2002
Manual Pre-disinfection/Cleaning and Sterilization Instructions for SATELEC wrenches
 Caution Manual Pre-disinfection/Cleaning and Sterilization Instructions for SATELEC wrenches Do not use steel wool or abrasive cleaners. Avoid solutions containing iodine or with a high chlorine content.
Caution Manual Pre-disinfection/Cleaning and Sterilization Instructions for SATELEC wrenches Do not use steel wool or abrasive cleaners. Avoid solutions containing iodine or with a high chlorine content.
Instructions for Use (GA16)
 Instructions for Use (GA16) Instruments and Accessories for Arthroscopy Endoscopy Laparoscopy Resectoscopy Urology Hysteroscopy made from stainless steels as per DIN EN ISO 7153-1 and from high quality
Instructions for Use (GA16) Instruments and Accessories for Arthroscopy Endoscopy Laparoscopy Resectoscopy Urology Hysteroscopy made from stainless steels as per DIN EN ISO 7153-1 and from high quality
Reprocessing of surgical instruments
 Reprocessing of surgical instruments Reprocessing of surgical instruments Reprocessing Reprocessing Content 1 2 5... Introduction 6 7... Pre-treatment Microsoft Tag Readers Thanks to the Microsoft Tag
Reprocessing of surgical instruments Reprocessing of surgical instruments Reprocessing Reprocessing Content 1 2 5... Introduction 6 7... Pre-treatment Microsoft Tag Readers Thanks to the Microsoft Tag
REUSABLE ENDOSCOPY INSTRUMENTS (thermo resistant)
 IMPORTANT ADVICES PLEASE READ CAREFULLY AND KEEP THE INSTRUCTIONS FOR FUTURE REFERENCE REUSABLE ENDOSCOPY INSTRUMENTS Field of application The following instructions are for all reusable endoscopy instruments
IMPORTANT ADVICES PLEASE READ CAREFULLY AND KEEP THE INSTRUCTIONS FOR FUTURE REFERENCE REUSABLE ENDOSCOPY INSTRUMENTS Field of application The following instructions are for all reusable endoscopy instruments
Read this Safety Guide first.
 LCD Projector User's Manual - Safety Guide Thank you for purchasing this projector. About The Symbols Read this Safety Guide first. Before using, read the user manuals for this projector to ensure correct
LCD Projector User's Manual - Safety Guide Thank you for purchasing this projector. About The Symbols Read this Safety Guide first. Before using, read the user manuals for this projector to ensure correct
Instructions for care, maintenance, cleaning and sterilization of Smith & Nephew orthopaedic devices
 Instructions for care, maintenance, cleaning and sterilization of Smith & Nephew orthopaedic devices 71381339 REVA CLEANING AND STERILIZATION BRO.indd 1 5/21/12 3:41 PM 71381339 REVA CLEANING AND STERILIZATION
Instructions for care, maintenance, cleaning and sterilization of Smith & Nephew orthopaedic devices 71381339 REVA CLEANING AND STERILIZATION BRO.indd 1 5/21/12 3:41 PM 71381339 REVA CLEANING AND STERILIZATION
Christina Bradley Laboratory Manager Hospital Infection Research Laboratory Queen Elizabeth Hospital Birmingham, UK
 Christina Bradley Laboratory Manager Hospital Infection Research Laboratory Queen Elizabeth Hospital Birmingham, UK Irrespective of where the decontamination is performed or who it is performed by the
Christina Bradley Laboratory Manager Hospital Infection Research Laboratory Queen Elizabeth Hospital Birmingham, UK Irrespective of where the decontamination is performed or who it is performed by the
TECHNOSKLO Ltd. Držkov
 TECHNOSKLO Ltd. Držkov Distillation apparatus for producing of distilled water TYPE DP 4000 Operating manual TECHNOSKLO Ltd. 468 24 Držkov 135 Czech Republic tel.: 00420 483 346 403 fax: 00420 483 385
TECHNOSKLO Ltd. Držkov Distillation apparatus for producing of distilled water TYPE DP 4000 Operating manual TECHNOSKLO Ltd. 468 24 Držkov 135 Czech Republic tel.: 00420 483 346 403 fax: 00420 483 385
SeedVac TM REF 90091
 WWW..COM SeedVac TM REF 90091 STANDARD IMAGING INC. 7601 Murphy Drive Middleton, WI 53562 Feb / 2005 2005 Standard Imaging Inc. DOC #80191-06 TEL 800.261.4446 TEL 608.831.0025 FAX 608.831.2202 www.standardimaging.com
WWW..COM SeedVac TM REF 90091 STANDARD IMAGING INC. 7601 Murphy Drive Middleton, WI 53562 Feb / 2005 2005 Standard Imaging Inc. DOC #80191-06 TEL 800.261.4446 TEL 608.831.0025 FAX 608.831.2202 www.standardimaging.com
GETINGE CLEAN DETERGENT RANGE GET MAXIMUM PERFORMANCE VALIDATED
 GETINGE CLEAN DETERGENT RANGE GET MAXIMUM PERFORMANCE Tested for optimal cleaning and performance ciso 15883 VALIDATED 2 GETINGE CLEAN GETINGE CLEAN THE ANSWER TO YOUR CLEANING PROBLEMS What does the word
GETINGE CLEAN DETERGENT RANGE GET MAXIMUM PERFORMANCE Tested for optimal cleaning and performance ciso 15883 VALIDATED 2 GETINGE CLEAN GETINGE CLEAN THE ANSWER TO YOUR CLEANING PROBLEMS What does the word
Cavitator Ultrasonic Cleaner Instruction Manual
 Cavitator Ultrasonic Cleaner Instruction Manual Made in China 1333 South Claudina Street Anaheim, CA 92805 Toll Free: (800) 854 9305 Tel: (714) 533 2221 FAX: (714) 635 7539 Web site: www.mettlerelectronics.com
Cavitator Ultrasonic Cleaner Instruction Manual Made in China 1333 South Claudina Street Anaheim, CA 92805 Toll Free: (800) 854 9305 Tel: (714) 533 2221 FAX: (714) 635 7539 Web site: www.mettlerelectronics.com
Instructions for Cleaning, Sterilization, Inspection and Maintenance. of Trauma & Extremities Medical Devices
 Instructions for Cleaning, Sterilization, Inspection and Maintenance of Trauma & Extremities Medical Devices Contents Page 1. Introduction 3 Warnings and Precautions 3 2. Processing Instructions 4 3. Cleaning
Instructions for Cleaning, Sterilization, Inspection and Maintenance of Trauma & Extremities Medical Devices Contents Page 1. Introduction 3 Warnings and Precautions 3 2. Processing Instructions 4 3. Cleaning
Attendance Sheet. (List each model reviewed during this session) Print Name Signature Title. Print Name Signature Title.
 THIS CHECKLIST IS DESIGNED FOR USE SOLELY AS A CUSTOMER EDUCATIONAL TOOL, AND IS NOT INTENDED TO REPLACE OR ANY WAY MODIFY THE OLYMPUS INSTRUCTION MANUAL/REPROCESSING MANUAL. BE SURE TO FOLLOW THE DETAILED
THIS CHECKLIST IS DESIGNED FOR USE SOLELY AS A CUSTOMER EDUCATIONAL TOOL, AND IS NOT INTENDED TO REPLACE OR ANY WAY MODIFY THE OLYMPUS INSTRUCTION MANUAL/REPROCESSING MANUAL. BE SURE TO FOLLOW THE DETAILED
QUICK 3202 OPERATION MANUAL. Lead Free Soldering Station
 QUICK 30 Lead Free Soldering Station OPERATION MANUAL Thank you for purchasing the unit. It is designed for lead free soldering. Please read this manual carefully before use and keep it for future reference.
QUICK 30 Lead Free Soldering Station OPERATION MANUAL Thank you for purchasing the unit. It is designed for lead free soldering. Please read this manual carefully before use and keep it for future reference.
UL U TR LTRASONIC S ONIC SCALE ALER PIEZO MINI
 ULTRASONIC SCALER PIEZO MINI CONTENTS XI - SYMBOLS 1. INTRODUCTION 1 Alternating current Type BF device 2. WARNINGS 1 3. PRESENTATION 1 3.1 Presentation 1 3.2 Technical description 2! Warning, please refer
ULTRASONIC SCALER PIEZO MINI CONTENTS XI - SYMBOLS 1. INTRODUCTION 1 Alternating current Type BF device 2. WARNINGS 1 3. PRESENTATION 1 3.1 Presentation 1 3.2 Technical description 2! Warning, please refer
OPERATION MANUAL. LED Coupling OM-TR355E. Please read this operation manual carefully and file for future reference.
 OPERATION MANUAL LED Coupling OM-TR355E Please read this operation manual carefully and file for future reference. Caution When operating the product always consider the safety of the patient. The product
OPERATION MANUAL LED Coupling OM-TR355E Please read this operation manual carefully and file for future reference. Caution When operating the product always consider the safety of the patient. The product
Additionally, consider the legal provisions valid for your country as well as to the hygienic instructions of the doctor s practice or hospital.
 Reprocessing of Dental Hand Instruments and Accessories 1.0 Fundamental points All instruments are to be cleaned, disinfected, and sterilized prior to each use. In addition, cleaning, disinfection and
Reprocessing of Dental Hand Instruments and Accessories 1.0 Fundamental points All instruments are to be cleaned, disinfected, and sterilized prior to each use. In addition, cleaning, disinfection and
LipoCollector II Operating Instructions
 1 of 26 LipoCollector II Operating Instructions ISO 13485 Operating instructions for LipoCollector II Item No. 650100 All rights reserved, particularly the right to reproduce and distribute as well as
1 of 26 LipoCollector II Operating Instructions ISO 13485 Operating instructions for LipoCollector II Item No. 650100 All rights reserved, particularly the right to reproduce and distribute as well as
Rapid Heat Sterilizers with 6, 8, and 12 Minute Sterilization Cycle Times
 Rapid Heat Sterilizers with 6, 8, and 12 Minute Sterilization Cycle Times USER MANUAL MODELS: COX 115V-N COX 220V-N COX 115V-R COX 220V-R 2364 Leicester Road, P.O. Box 175, Leicester, NY 14481 Phone (585)
Rapid Heat Sterilizers with 6, 8, and 12 Minute Sterilization Cycle Times USER MANUAL MODELS: COX 115V-N COX 220V-N COX 115V-R COX 220V-R 2364 Leicester Road, P.O. Box 175, Leicester, NY 14481 Phone (585)
4/8/2014 GOAL PATIENT EQUIPMENT
 Module G APPLICATION OF CLEANING, DISINFECTION AND STERILIZATION PRINCIPLES TO PATIENT CARE EQUIPMENT IN OUTPATIENT HEALTHCARE SETTINGS GOAL Understand how to apply the principles of disinfection and sterilization
Module G APPLICATION OF CLEANING, DISINFECTION AND STERILIZATION PRINCIPLES TO PATIENT CARE EQUIPMENT IN OUTPATIENT HEALTHCARE SETTINGS GOAL Understand how to apply the principles of disinfection and sterilization
Cavitator Ultrasonic Cleaner Instruction Manual Model 20G
 Cavitator Ultrasonic Cleaner Instruction Manual Model 20G Made in China 1333 South Claudina Street Anaheim, CA 92805 Toll Free: (800) 854 9305 Tel: (714) 533 2221 FAX: (714) 635 7539 Web site: www.mettlerelectronics.com
Cavitator Ultrasonic Cleaner Instruction Manual Model 20G Made in China 1333 South Claudina Street Anaheim, CA 92805 Toll Free: (800) 854 9305 Tel: (714) 533 2221 FAX: (714) 635 7539 Web site: www.mettlerelectronics.com
Invacare Propad. General. Cushion User Manual. General information. Warranty. Standards and regulations. Symbols in this user manual.
 Invacare Propad Revolve / Revolveˢⁱ en Cushion User Manual General General information IenI Repositioning regularly is recommended to manage the risk for and aid in the prevention of skin breakdown. Essential
Invacare Propad Revolve / Revolveˢⁱ en Cushion User Manual General General information IenI Repositioning regularly is recommended to manage the risk for and aid in the prevention of skin breakdown. Essential
Manual. Reprocessing. RICHARD WOLF Heat-Stable Instruments. GA-J020 / en / V17.0 / PDI
 Manual Reprocessing of RICHARD WOLF Heat-Stable Instruments GA-J020 / en / 2014-05 V17.0 / PDI 13-6891 GERMANY RICHARD WOLF GmbH 75438 Knittlingen Pforzheimerstr. 32 Telephone: +49 70 43 35-0 Telefax:
Manual Reprocessing of RICHARD WOLF Heat-Stable Instruments GA-J020 / en / 2014-05 V17.0 / PDI 13-6891 GERMANY RICHARD WOLF GmbH 75438 Knittlingen Pforzheimerstr. 32 Telephone: +49 70 43 35-0 Telefax:
Important information
 Version SE_023827 AK Date March 2015 Important information (with Cleaning and Sterilization Instructions) Important information 2 Basic Instructions on the Use of Synthes Implants and Instruments for Orthopedics
Version SE_023827 AK Date March 2015 Important information (with Cleaning and Sterilization Instructions) Important information 2 Basic Instructions on the Use of Synthes Implants and Instruments for Orthopedics
Glass Chimney Hood. Installation & User Instructions Please keep for future reference
 Glass Chimney Hood Installation & User Instructions Please keep for future reference 4897549 4897556 Important Please read these instructions fully before installing or using These instructions contain
Glass Chimney Hood Installation & User Instructions Please keep for future reference 4897549 4897556 Important Please read these instructions fully before installing or using These instructions contain
Introduction. Table of Contents
 HYDROGIZE MANUAL Introduction Thank you for purchasing HydroGize, Young Living s hydrogen generator. Please read this manual carefully to ensure safe and easy use. Table of Contents 1. Table of Contents...
HYDROGIZE MANUAL Introduction Thank you for purchasing HydroGize, Young Living s hydrogen generator. Please read this manual carefully to ensure safe and easy use. Table of Contents 1. Table of Contents...
Instructions for Use: Endoplus Modular Handle Assemblies, Multi-Use Scissors Inserts, Reusable Inserts
 Modular Slide Lock Handle Assembly 1 Movable Handle 2 Fixed Handle 3 Handle Access For Cleaning 4 Window 5 Handle Screw 6 Keeper 7 Nonconductive Luer Port Cap (shown covering the Luer Port) 8 Shaft Insulation
Modular Slide Lock Handle Assembly 1 Movable Handle 2 Fixed Handle 3 Handle Access For Cleaning 4 Window 5 Handle Screw 6 Keeper 7 Nonconductive Luer Port Cap (shown covering the Luer Port) 8 Shaft Insulation
MODEL 7000 SUCTION UNIT
 MODEL 7000 SUCTION UNIT OPERATOR S MANUAL Caution Federal law restricts this device to sale by or on order of a physician, or any other practitioner licensed by the law of the State in which he practices
MODEL 7000 SUCTION UNIT OPERATOR S MANUAL Caution Federal law restricts this device to sale by or on order of a physician, or any other practitioner licensed by the law of the State in which he practices
OPERATING INSTRUCTIONS MEDAP CAP PLUGS
 OPERATING INSTRUCTIONS MEDAP CAP PLUGS Subject to technical modification! Illustrations and technical specifications may vary slightly from those in these Operating Instructions as a result of ongoing
OPERATING INSTRUCTIONS MEDAP CAP PLUGS Subject to technical modification! Illustrations and technical specifications may vary slightly from those in these Operating Instructions as a result of ongoing
ENDOSCOPE REPROCESSOR OER-Pro Operation Manual
 Chapter 5 End-of-Day Checks Chapter 5 End-of-Day Checks To ensure safe, reliable operation, inspect and clean all parts of the device regularly. Check Checks at the end of every working day 5.1 Turning
Chapter 5 End-of-Day Checks Chapter 5 End-of-Day Checks To ensure safe, reliable operation, inspect and clean all parts of the device regularly. Check Checks at the end of every working day 5.1 Turning
The Principles, The Process, and The Results:
 The Principles, The Process, and The Results: Presented by: Fred Alston, CSPDT Eastern Region Clinical Sales and Support Manager April 21, 2017 I am an employee of Healthmark Industries Fraser, Michigan
The Principles, The Process, and The Results: Presented by: Fred Alston, CSPDT Eastern Region Clinical Sales and Support Manager April 21, 2017 I am an employee of Healthmark Industries Fraser, Michigan
User Instruction &)+ 4: CFH110QB
 User Instruction &)+ 4: CFH110QB 10 Safety Information In the interest of your safety and to ensure the correct use, before installing and first using the appliance, read this user manual carefully, including
User Instruction &)+ 4: CFH110QB 10 Safety Information In the interest of your safety and to ensure the correct use, before installing and first using the appliance, read this user manual carefully, including
Endo Optiks E2 Laser Endoscopy System Contents:
 Endo Optiks E2 Laser Endoscopy System Contents: Page 1: Quick Setup Guide Page 3: Endoscope Handling Guide Page 5: Troubleshooting: E2 and Laser Endoscopy System QUICK SETUP GUIDE: E2 Laser and Endoscopy
Endo Optiks E2 Laser Endoscopy System Contents: Page 1: Quick Setup Guide Page 3: Endoscope Handling Guide Page 5: Troubleshooting: E2 and Laser Endoscopy System QUICK SETUP GUIDE: E2 Laser and Endoscopy
Visao HIGH-SPEED OTOLOGIC DRILL CATALOG
 Visao HIGH-SPEED OTOLOGIC DRILL CATALOG Visao High-Speed Otologic Drill with the Integrated Power Console (IPC ) System High speed and torque 80,000 rpm max speed provides rapid, yet precise bone removal
Visao HIGH-SPEED OTOLOGIC DRILL CATALOG Visao High-Speed Otologic Drill with the Integrated Power Console (IPC ) System High speed and torque 80,000 rpm max speed provides rapid, yet precise bone removal
Operator s Manual. Model G14TC-3 Disinfection Soak Station for Transesophageal Ultrasound Probes
 Model G14TC-3 Disinfection Soak Station for Transesophageal Ultrasound Probes Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741 Fax: 1-877-329-2482
Model G14TC-3 Disinfection Soak Station for Transesophageal Ultrasound Probes Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741 Fax: 1-877-329-2482
OPERATING INSTRUCTIONS MEDAP MEDICAL GAS DISTRIBUTOR FOR DIN STANDARD
 OPERATING INSTRUCTIONS MEDAP MEDICAL GAS DISTRIBUTOR FOR DIN STANDARD Subject to technical modification! Illustrations and technical specifications may vary slightly from those in these Operating Instructions
OPERATING INSTRUCTIONS MEDAP MEDICAL GAS DISTRIBUTOR FOR DIN STANDARD Subject to technical modification! Illustrations and technical specifications may vary slightly from those in these Operating Instructions
FEATURES. Time set: 0~30 minutes digital timer; Temperature adjustable 20~80 digital control, Material: good quality stainless steel SUS304,
 FEATURES Time set: 0~30 minutes digital timer; Temperature adjustable 20~80 digital control, Material: good quality stainless steel SUS304, Drainage system: styles with 6L above was installed drainage
FEATURES Time set: 0~30 minutes digital timer; Temperature adjustable 20~80 digital control, Material: good quality stainless steel SUS304, Drainage system: styles with 6L above was installed drainage
WiseBath WSB Shaking Water Bath Operation Manual (Version 1.4.0)
 WiseBath WSB Shaking Water Bath Operation Manual (Version 1.4.0) written for Model WSB-18/-30/-45 Contents Page 1. Safety Instructions........ 3 2. Before Use...... 8 3. Introduction... 9 4. Package Contents...
WiseBath WSB Shaking Water Bath Operation Manual (Version 1.4.0) written for Model WSB-18/-30/-45 Contents Page 1. Safety Instructions........ 3 2. Before Use...... 8 3. Introduction... 9 4. Package Contents...
