United States Patent (19) Wheldon
|
|
|
- Steven Cummings
- 5 years ago
- Views:
Transcription
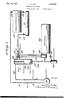
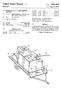
1 United States Patent (19) Wheldon 11 Patent Number: (45. Date of Patent: Sep. 6, METHOD FOR REMOVING WATER FROM ETHANOL 75 Inventor: Alfred G. Wheldon, Essex, Great Britain 73) Assignee: United Distillers P.L.C., Scotland 21 Appl. No.: 4, Filed: Jan., Foreign Application Priority Data Jan. 17, 1986 GB United Kingdom Int. Cl."... B01D3/14; B01D 11/04; B01D 15/00; C12P 7/06 52 U.S. C.... 3/19; 3/26; 3/27; 3/41; 3/46; 3/49; 3/71; 3/DIG. 4; 3/DIG. 8; 3/DIG. 13; 426/494; 435/161; 568/916 58) Field of Search... 3/49, 19, DIG. 13, 3/41, 74, 77, 84, 71, DIG. 8, DIG. 4, 26, 21, 27, 43 46; 435/161; 426/493, 494; 568/916, 918, 917 (56) References Cited U.S. PATENT DOCUMENTS 4,273,621 6/1981 Fornoff... 3/19 4,327,184 4/1982 Johnson et al /DIG. 13 4,349,415 9/1982 De Filippi et al... 3/49 4,359,593 11/1982. Feldman /916 4,4,561 12/1983 Chen /61 4,465,875 8/1984 Greenbank et al /96 4,487,614 12/1984 Yon... 3/19 4,492,808 1/1985 Hagen et al /98 4,522,9 6/1985 Thorsson et al.... 4,556,460 12/1985 Robertson et al.... 2O3/49 FOREIGN PATENT DOCUMENTS /1984 European Pat. Off /1984 European Pat. Off.. Primary Examiner-Wilbur Bascomb Attorney, Agent, or Firm-Sughrue, Mion, Zinn, Macpeak and Seas 57 ABSTRACT A method and apparatus for removing water from a liquid mixture of water and ethanol contacts it with liquid carbon dioxide so that the ethanol is preferen tially transferred into solution, dries the solution using an adsorbent, and then recovers dry ethanol by distill ing off the carbon dioxide. This process is particularly energy efficient especially when it includes a fermenta tion process to generate the ethanol and uses the carbon dioxide generated during the fermentation as the source of liquid carbon dioxide. In this case the method and apparatus provide an additional product of dry carbon dioxide. Claims, 3 Drawing Sheets DISTILLATION EXTRACTION O PRIMARY SEPARATION SECONDARY SEPARATION FERMENTED WASH CARBON OOXIDE FERMENTER GAS COMPRESSION & QUEFACTION AIR FOR ORYER REGENERATION LIQUEFACTION FRACTIONAL DISTILLATION PURE ANYHOROUS ETHANOL FRACTIONATED FUSELOILS PURE CO2 NEAR ANHYDROUS ETHANOL & CONGENERS GASOHO
2
3 U.S. Patent LZ 01
4 U.S. Patent Sep. 6, 1988 Sheet 3 of 3 Fig. 3 PURGE GAS FROM HEAT EXCHANGER 3 ARICO2 ARICO2 ARICO2 ORAN ORAN ORAN
5 1. METHOD FOR REMOVING WATER FROM ETHANOL BACKGROUND OF THE INVENTION This invention relates to a method and apparatus for removing water from a mixture containing both water and ethanol. At present water is usually removed from such a mixture by distillation but ethanol forms a binary azeo trope with water and consequently, by using simple distillation techniques, it is impossible to remove all of the water. When producing a potable spirit azeotrope strength spirit is the maximum ethanol concentration that is produced. Water is removed from such a binary azeotrope to produce a substantially water-free ethanol for industrial use by adding a third component to pro duce a ternary system. Upon subsequent distillation of the ternary system substantially water-free ethanol is produced. To obtain substantially anhydrous ethanol using this technique requires a substantial quantity of energy and, in the past, a number of proposals have been made to remove the water more efficiently. For example, it is known to treat an azeotrope mixture with a desiccant such as fused sodium or potassium acetate and thereby remove water from such a mixture. It is also known to use a molecular sieve dryer operating in either the liquid or the vapour phase to remove the last remaining traces of water from substantially water-free ethanol but, in general, the use of a molecular sieve has been confined to such a polishing role. In addition to these conventional techniques specific proposals are described in U.S. Pat. Nos. 3,132,079, 4,273,621 and EP-A-No involving the use of a molecular sieve to remove water from an azeotrope vapour resulting from distillation and containing an organic liquid and water. In the first of these a method is discussed in which a molecular sieve is used to adsorb water from a vapour phase azeotrope mixture of water and isopropanol. To regenerate the molecular sieve some of the water-free isopropanol, so produced is heated and passed through the molecular sieve in the reverse direction to remove water from the water satu rated molecular sieve. This wet isopropanol is then returned to the distillation system. This conserves the isopropanol but the system is inefficient and uses a great deal of energy firstly in the distillation of the mixture to obtain the vapour phase azeotrope and secondly to heat the dry isopropanol so produced and use this to regen erate the molecular sieve. This results in wetting the isopropanol again and hence reducing the yield of dry isopropanol whilst, at the same time, requiring the use of still further energy to redistil the re-wetted isopropa nol. In the second of these proposals, a system for remov ing water from a vapour phase ethanol/water azeotrope is described in which an ethanol/water mixture is sub jected to high pressure distillation at a pressure of 7.5 bar. The resulting vapour phase azeotrope is then di luted with a carrier gas consisting of carbon dioxide or nitrogen and passed through a molecular sieve to re move the water vapour. The ethanol is allowed to con dense out and the carrier gas is used in the regeneration of the water saturated molecular sieve. By careful choice of operating temperatures and pressures and also using the heat of adsorption and desorption it is possible to use very little energy for the removal of the water from the vapour phase azeotrope but, of course, the high pressure distillation part of the process does re quire a considerable amount of energy. Moreover, this system is entirely concerned with the removal of water from the vapour and not only relies on it being a vapour but on it being a vapour resulting from a high pressure distillation system. This system could not be applied to a liquid feed stock unless that liquid feed stock was vaporized initially and this would also require consider able quantities of energy. In the third of these a process is described in which a carbon molecular sieve is used to remove water from an ethanol/water azeotrope vapour and it is postulated that such molecular sieves could also be used to remove water from ethanol in the liquid phase. Other proposals have been made, for example in EP A-No and U.S. Pat. No. 4,4,561 to adsorb ethanol onto a molecular sieve material to remove it from an ethanol/water mixture and then recover the ethanol upon regenerating the molecular sieve material. SUMMARY OF THE INVENTION According to a first aspect of this invention a method of removing water from a mixture containing water and ethanol comprises the steps of: (a) contacting a liquid ethanol water mixture with liquid carbon dioxide so that the ethanol is preferen tially transferred from the mixture into solution with the liquid carbon dioxide to increase the ratio of ethanol to water in the liquid carbon dioxide; (b) supplying heat to the mixture containing ethanol and carbon dioxide to vaporize it and thereby increase the proportion of ethanol in the mixture and concen trate it; (c) scrubbing the vapour evolved from step (b) with the mixture fed to step (b) to remove substantially all of the ethanol from the vapour evolved in step (b); (d) condensing the evaporated carbon dioxide vapour and recycling the reformed liquid carbon dioxide to return it to the contaction step (a); (e) continuing the recycling of the reformed liquid carbon dioxide to increase the concentration of ethanol and so produce a concentrated mixture; (f) drying the combined mixture resulting from step (a) or the concentrated mixture resulting from step (e) by a process including contacting the mixture with an adsorbent which adsorbs substantially all the water from it; (g) feeding the concentrated dry mixture containing ethanol and carbon dioxide to a distillation column which is cooled at the top and heated at the base to recover substantially water free ethanol at the base. According to a second aspect of this invention a plant for removing water from a mixture containing water and ethanol comprises: a contaction column having a first inlet for the mix ture containing ethanol and water, a second inlet for liquid carbon dioxide below the first inlet, a first outlet for the stripped mixture below the second inlet, and a second outlet for a solution of ethanol and liquid carbon dioxide above the first inlet; first heat exchange means having a liquid inlet, a liquid outlet and a vapour outlet; second heat exchange means having a vapour inlet and a liquid outlet; a tailing column having a liquid inlet and outlet and a vapour inlet and outlet, the vapour outlet from the tailing column being connected to the vapour inlet of
6 3 the second heat exchange means, the liquid outlet of the tailing column being connected to the liquid inlet of the first heat exchange means and the vapour outlet of the first heat exchange means being connected to the va pour inlet of the tailing column; means connected between the liquid outlet of the second heat exchange means and the second inlet of the liquid-liquid contaction column; distillation column having a liquid inlet; and, a dryer including an adsorbent material, the dryer having an inlet and an outlet and being connected in series between the second outlet of the contaction col umn and the liquid inlet of the tailing column or con nected between the liquid outlet of the first heat ex change means and the liquid inlet of the distillation column; the distillation column having a vapour outlet located at its top and a liquid outlet at its base from which water free ethanol is recovered. Preferably the ethanol content of the ethanol/water mixture is as high as possible and is at least 40% or 60% w/w and more preferably at least 70% w/w. It is espe cially preferred that the ethanol content of the mixture is substantially 80% w/w. Preferably the solution of ethanol and liquid carbon dioxide leaving the contac tion step has its ratio of ethanol to water increased to at least 9: during the contaction step. This reduces the amount of water to be removed by the dryer and hence reduces the energy required to regenerate the molecular sieve. Preferably the concentration of ethanol in the substantially water-free mixture is increased in step (e) until it is present at at least % w/w. Typically a fermented wash has an ethanol content of between 6% and 12% w/w. Before such a wash can economically have water removed from it by a method and apparatus in accordance with this invention the fermented wash must be subjected to an initial concen tration process. Similarly the product obtained by the industrial synthesis of ethanol is an ethanol water mix ture having a low concentration of ethanol. Accord ingly such mixtures should also be subject to an initial concentration process. The initial concentration process may have the form of a simple distillation carried out in a wash still which strips substantially all of the ethanol from the fermented wash or it may include some rectification and reflux stages to increase the ethanol content to a higher level and typically to between 70 and 80% w/w of ethanol. When the ethanol is obtained by fermentation it is also possible to provide a continuous fermentation and pri mary distillation step in which a continuous fermenta tion process is employed with a substrate to be fer mented being introduced continuously into a fermenter and the resulting fermented wash being passed through a distillation column providing an output of between 30% and 40% ethanol w/w. A part of the stripped wash is then returned to the fermenter and the remainder is concentrated and discharged as stillage. The energy costs for an initial concentration step using conventional distillation techniques or a continu ous fermentation distillation process are not great. The energy costs of conventional distillation processes only increase substantially when they are used to increase the ethanol concentration to more than 80% w/w, as shown in Table 1 below. 5 O TABLE 1 Energy required to increase ethanol concentration from x% to y2 by weight X y KJ/Kg O 60 3,100 O 70 3,0 O 80 3,300 O 90 3, O ,400 6,300 By connecting the dryer in series between the liquid outlet of the first heat exchange means and the liquid inlet of the distillation column and hence carrying out the drying step (f) on the concentrated mixture resulting from step (e) less water has to be removed by the dryer. This results from the mixture of ethanol and carbon dioxide being concentrated at this point. As a result of this the size and capacity of the dryers can be reduced by about ten percent. However, connecting the dryer in series between the second outlet of the contaction col umn and the liquid inlet of the tailing column and hence carrying out the drying step (f) on the mixture resulting from the contaction step (a) has the advantage that all the apparatus downstream of the dryers can then be made of mild steel instead of stainless steel and this reduces considerably the cost of the plant, Preferably the means connected between the liquid outlet of the second heat exchange means and the sec ond inlet of the liquid-liquid contaction column include a hold-up tank in which the condensed liquid carbon dioxide collects and from which the condensed liquid carbon dioxide is taken to the second inlet of the liquid liquid contaction column. A cooler may be connected between the outlet of the hold-up tank and the second inlet of the liquid-liquid contaction column to ensure that the liquid carbon dioxide is at a temperature below its equilibrium point at the pressure subsisting in the contaction column. This ensures that the carbon dioxide remains in a liquid state during its flow through the liquid-liquid contaction column. The liquid-liquid con taction column may be, for example, a sieve plate col umn, a packed column, a bubble-cap column, a falling film column, a spray column or a disc and doughnut column. It is preferred that the contaction step between the mixture containing ethanol and water and the liquid carbon dioxide is carried out at a temperature above 10 C. Above 10 C. there is sufficient difference in density between the ethanol and water and the liquid carbon dioxide to enable a very effective contaction and sepa ration to take place. Another advantage of working above this temperature is that formation of carbon diox ide hydrate CO2.8H2O is prevented. This carbon diox ide hydrate is a solid which can inhibit the flow in the contaction column. The first and second heat exchange means may be opposite sides of a common heat exchanger. In this case a carbon dioxide vapour compressor is connected be tween the vapour outlet of the tailing column and the vapour inlet of the second heat exchange means. With this arrangement the heat of vaporisation required to vaporise liquid carbon dioxide in the first heat exchange means is provided mainly by the heat of liquefaction of carbon dioxide vapour evolved in the second heat ex change means as described fully in our earlier specifica tion GB-A-No This arrangement is particu
7 5 larly energy efficient but the temperatures subsisting in the first and second heat exchange means are naturally linked together taking into account the capacity and performance of the compressor. Alternatively the first and second heat exchange means may be formed by separate first and second heat exchangers each having a primary path for a warm vapour medium to be cooled and liquefied and a sec ondary path for a cool liquid medium to be warmed and vaporized. In this case preferably the primary path of the first heat exchanger is connected in a loop with the secondary path of the second heat exchanger with a compressor on one side of the loop and an expansion valve on the other side of the loop to provide a heat pump system with a working fluid such as an haloge nated hydrocarbon, for example dichlorodifluorometh ane. In this case it is the heat of liquefaction of the work ing fluid which provides the heat required to evaporate the carbon dioxide vapour from the secondary path of the first heat exchanger and the cooling caused by evap oration of the working fluid at a lower pressure and therefore temperature which condenses the carbon di oxide vapour in the primary path of the second heat exchanger. This system may use a little more energy than the first system described although it is still energy efficient but this extra energy usage is offset by the advantages gained by being able to decouple the tem peratures of the heat exchange surfaces in the first and second heat exchangers. When the apparatus includes a fermenter it is pre ferred that the carbon dioxide produced during the fermentation of, for example, a cereal product, is used to provide the liquid carbon dioxide used in accordance with this invention, and it is preferred that dry substan tially pure carbon dioxide is produced as an additional product of this invention by recovering it as a top prod uct from the distillation column producing dry ethanol as its bottom product. In this case a carbon dioxide outlet from the fermenter is fed to a carbon dioxide compressor and then the condensed carbon dioxide is fed to the second inlet of the contaction column. The dryer may also include an initial pervaporation dryer in which water is removed using a preferentially permeable membrane. However it is preferred that the dryer consists solely of the adsorbent material. It is preferred that the adsorbent material has a pore aper ture size of substantially 3 Angstroms (0.3 nm). Also it is preferred that the adsorbent material is a crystalline zeolite. Preferably the dryer consists of at least two and pref. erably four chambers arranged in parallel so that the full flow of the combined mixture leaving the contaction column or the concentrated mixture leaving the liquid outlet of the first heat exchanger passes through one of the chambers to have the water removed from it whilst the adsorbent material in the other, or others, of the chambers is regenerated. The recompressed and recon densed carbon dioxide may be used to flush the ethanol rich carbon dioxide from the dryer before its regenera tion. Preferably the outlet of the compressor which com presses the carbon dioxide from the fermenter is fed to a heat exchanger in which it is cooled. Heat from the compressed carbon dioxide gas is used to heat air in the heat exchanger and this hot air is then used to regener ate the adsorbent material in the dryer. The compressed carbon dioxide is also passed through a cooler before being fed to the contaction column. 6 Preferably the raffinate leaving the second outlet of the liquid-liquid contaction column is fed to a raffinate degasser which separates the carbon dioxide from the spent liquid. This carbon dioxide is preferably intro 5 duced into the flow of carbon dioxide leaving the fer menter and is then compressed and recirculated. Prefer ably the degassed raffinate is returned to the fermenter to be used again in the fermentation. Where the initial mixture containing ethanol and 10 water is produced by a fermentation process the mix ture typically includes congeners such as fusel oils and higher alcohols. The congeners tend to carry through the method and apparatus in accordance with this in vention and so are present in the final anhydrous etha 15 nol output. Where the anhydrous ethanol product pro duced by the method and apparatus in accordance with this invention is intended to be used as a liquid fuel, or a liquid fuel additive, the presence of the congeners and is irrelevant and thus the product obtained from the base of the distillation column can be used directly for such purposes. However, where the anhydrous ethanol product is required for potable purposes or where it is required to obtain substantially pure ethanol the output obtained from the base of the stripping column is prefer ably subjected to a fractional distillation process to separate the ethanol from the congeners. Substantially pure anhydrous ethanol can be readily separated from the congeners by this fractional distillation process 30 since water is absent. When this further distillation step is carried out in a single fractionating column there is inevitably some carry over of the higher or lower boil ing point fractions. For some purposes such as for use in the fortification of wines this may be satisfactory. How 35 ever, it is preferred that separate topping and tailing columns are used to provide substantially pure anhy drous ethanol. BRIEF DESCRIPTION OF THE DRAWINGS 40 Examples of a method and apparatus in accordance with the present invention will now be described with reference to the accompanying drawings, in which: FIG. 1 is a diagram of a first example of the appara tus; 45 FIG. 2 is a diagram of part of a second example of the apparatus; and, FIG. 3 is a diagram of the dryer. DESCRIPTION OF PREFERRED EXAMPLE 50 A carbohydrate feedstock is fermented by yeast in a fermenter 1 to a product containing up to 12% w/w ethanol but more typically 6% w/w ethanol. The car bon dioxide evolved from the fermentation process is compressed in a compressor 2 and then cooled in a heat 55 exchanger 3 followed by a cooler 4 to liquefy it. This fermented wash is then distilled in a simple distillation plant 5 to produce an ethanol water mixture which typically contains about 80% by weight of ethanol. This mixture is pumped by a pump 6 into a first inlet 7 of a contaction column 8. Contaction column 8 is a sieve plate column having twenty-five plates made from stainless steel. The contaction column 8 typically oper ates at a pressure of 59 bar and at a temperature of 15 C. Liquid carbon dioxide from the cooler 4 downstream 65 of the compressor 2 is introduced into a second inlet 9 located at the base of the contaction column 8 and passes upwards through the contaction column 8 in counter-current to the ethanol water mixture.
8 7 Ethanol and the congeners are preferentially taken into solution with the liquid carbon dioxide and a car bon dioxide solution rich in ethanol leaves a first outlet 10 in the top of the contaction column 8. The typical composition at this point is 90% w/w carbon dioxide, 9.3% w/w ethanol plus congeners, and 0.7% w/w wa ter. The raffinate from the contaction column 8 is essen tially water with small amounts of alcohol and carbon dioxide in solution. This is depressurised on entry to a vapour separator 11 from which the carbon dioxide is returned to the process via the fermentation gas com pressor 2. The aqueous solution is returned to either the primary distillation or to the fermentation system. The first outlet 10 at the top of the contaction column 8 is connected to a dryer system 12 shown in more detail in FIG. 3, consisting of four molecular sieve dryers 13, 14, 15 and 16 connected in parallel. The dryers are all packed with a crystalline zeolite having a nomi nal pore aperture size of 3 Angstroms (0.3 nm) such as type 3A manufactured by Laporte Industries plc of Luton, Bedfordshire. Typically the crystalline zeolite is present as 1 to 2 mm spheres. The dryers typically oper ate at a temperature of 15 C. and at an inlet pressure of 59 bar. The molecular sieve material adsorbs water to the extent of about % of its own weight before it requires regeneration. At any instant only one of the dryers is con nected in series with the flow of carbon dioxide to ad sorb water from the solution of ethanol and carbon dioxide. The other dryers are being regenerated or in a regenerated condition standing by to take over. The outlet from the dryer system 12 leads, via an analyser R to a flow control valve 17 and thence to an inlet 18 to a tailing column 19. The analyser R monitors the flow for the presence of water and, upon detection of water, changes from one to the other of the molecular sieve dryers The tailing column 19 is a packed column packed with half inch (12.5 mm) mild steel pall rings which typically operates at a temperature of 18 C. and a pres sure of 54 bar. The carbon dioxide ethanol solution then leaves a liquid outlet in the base of the column 19 and is led to a liquid inlet 21 in the shell side of an inclined shell and tube heat exchanger 22. The tailing column 19 and the heat exchanger 22 form a simple distillative system in which the ethanol concentration is increased from approximately 9% w/w to approximately 30% w/w. Typically the heat exchanger 22 is also made from mild steel. The carbon dioxide and ethanol solution is evaporated in the shell side of the heat exchanger 22 and the resulting vapour leaves a vapour outlet 23 in the shell side of the heat exchanger 22 and is taken to a vapour inlet 23 of the tailing column 19. In the tailing column 19 the flow of liquid carbon dioxide and ethanol scrubs the vapour leaving the shell side of the heat exchanger 22 to reduce the ethanol content so that the vapour leaving a vapour outlet in the top of the tailing column 19 contains substantially only carbon dioxide. This carbon dioxide vapour is fed to a compressor 24 which is typically a single stage reciprocating compres sor. The compressed gas is then fed into the tube side of the heat exchanger 22. The carbon dioxide vapour is heated during its recompression in compressor 24 and thus, when the recompressed carbon dioxide vapour is reintroduced into the tube side of the heat exchanger 22 it gives up both sensible and latent heat to cause evapo ration of carbon dioxide and ethanol solution in the shell O side of the heat exchanger 22. Meanwhile the com pressed carbon dioxide vapour recondenses to form liquid carbon dioxide which leaves the tube side of the heat exchanger 22 and is received in a hold-up tank. The hold-up tank typically operates at a pressure of the order of 67 bar and at a temperature of around 27 C. Liquid carbon dioxide from the hold-up tank is taken via the heat exchanger 26 which cools the carbon dioxide before introducing it through the second inlet 9 in the base of the contaction column 8. The degree of cooling exerted by the cooler 4 and by the heat ex changer 26 is controlled by temperature controllers to ensure that the carbon dioxide introduced into the con taction column 8 is below its boiling point and at the required extraction temperature. A liquid outlet from the shell side of the heat ex changer 22 feeds the ethanol carbon dioxide solution which is rich in ethanol and typically has an ethanol concentration of around 30% w/w into a distillation column 27. The distillation column 27 is typically a packed column packed with quarter inch (6 mm) mild steel Raschig or Lessing rings having a heat pump sys tem 28 connected to its base and top to provide reflux and boil-up. The heat pump system 28 typically uses an halogenated hydrocarbon such as dichlorodifluoro methane. In this secondary system carbon dioxide of high purity is obtained from the head of the distillation column 27 and ethanol containing less than 0.5% water w/w is obtained from the base. The pressure of the secondary system is generally less than that of the pri mary system since this facilitates flow from the primary to secondary system. The ease of separation of the etha nol plus congeners and the carbon dixoide is also im proved at the lower pressures. The high purity carbon dioxide from the head of the column 27 is suitable for sale for most purposes without further purification treatment. A proportion is recycled to the process by means of a pump 28. The substantially anhydrous mixture of ethanol and congeners is expanded from the base of the column 27 to a vapour/liquid separator 29. The liquid phase from this separator is passed to a fractional distillative unit which produces pure anhydrous ethanol and, fraction ated fusel oils. In a modification of this example the dryer 12 is con nected in series between the liquid outlet from the shell side of the heat exchanger 22 and the distillation column 27 as shown by dotted lines in FIG. 1. In this modifica tion the first outlet 10 in the top of the contaction col umn 8 is connected directly to the inlet 18 of the tailing column 19. In this case, since the solution containing ethanol is more concentrated the capacity of each of the dryers can be reduced by about 10%. Also, since the pressure is lower at this point the dryers can be less robustly constructed. However, with the dryer system 12 located in this position all of the heat exchanger 22 and tailing column 19 have to be made of stainless steel. To regenerate the dryer system 12 firstly the dryer to be regenerated, say dryer 13, is isolated from the flow of carbon dioxide solution leaving the contaction column 8 and this flow is fed via the dryer 14. All of the liquid from the dryer system 12 is drained and then it is con nected to regenerated dryer 16 to pressurise this with carbon dioxide. The dryer system 12 is then depressu rised. Air which has been heated in the heat exchanger 3 is fed to the dryer system 12 to desorb and flush out the water. The flow of air may be heated further in a
9 booster heater (not shown) if sufficient heat is generated by the compression of carbon dioxide. The easy drain ing of the liquid carbon dioxide/ethanol/water mixture from the bed is important as it results in negligible reten tion of ethanol in the bed at the commencement of the regeneration. After all the water has been removed the flow of hot air is stopped and then the bed of crystalline zeolite is cooled by flushing with cool air. The cool air after becoming heated by the hot bed may be used to start regeneration of the next dryer 14. After the dryer system 12 is cool it is flushed with carbon dioxide from the next dryer 13 to be regenerated to remove the air and then repressurized to its normal operating pressure. It then stands by to receive the carbon dioxide solution. This process is repeated on each dryer in turn when it requires regeneration. The use of a multiplicity of dry ers enables the process to proceed without interruption. The second example is identical to the first example with the exception of the parts shown in FIG. 2 which are used instead of the heat exchanger 22 and compres sor 24. In the second example two separate heat ex changers 30 and 31 are provided and these are con nected in a closed loop with an halogenated hydrocar bon heat pump system. The heat pump comprises a compressor 32 and an expansion valve 33 and the work ing fluid is typically dichlorodifluoromethane. This is compressed by the compressor 32 and the heat liberated by the condensation of the working fluid in the heat exchanger 30 provides the heat required to evaporate carbon dioxide from the mixture leaving the molecular sieve dryer 12. Evaporation of the working fluid at a lower pressure and therefore temperature after passing through the valve 33 results in condensation of the carbon dioxide vapour in the heat exchanger 31. This system has the advantage that the temperatures of the heat exchangers 30 and 31 and hence of the heat ex change surfaces used to provide heat to the carbon dioxide ethanol mixture and take heat from the carbon dioxide vapour are independent of one another. I claim: 1. A method of removing water from a mixture con taining water and ethanol comprising the steps of: (a) contacting a liquid ethanol water mixture with liquid carbon dioxide as a contaction step whereby the ethanol is preferentially transferred from said liquid ethanol water mixture into solution with said liquid carbon dioxide to increase the ratio of etha nol to water in said liquid carbon dioxide and pro vide a first fraction comprising ethanol/water and a second fraction comprising ethanol/water/car bon dioxide; (b) drying said second fraction comprising ethanol/- water/carbon dioxide resulting from step (a) to produce a dry mixture comprising ethanol and carbon dioxide by a process including contacting said combined mixture with an absorbent which adsorbs substantially all of said water from it; (c) supplying heat to said dry mixture comprising ethanol and carbon dioxide to cause volatilization of a fraction rich in carbon dioxide and removing said carbon dioxide rich fraction to thereby in crease the proportion of ethanol in the remaining dry mixture; (d) scrubbing the carbon dioxide rich vapour evolved in step (c) with said dry mixture to remove substan tially all of said ethanol from the carbon dioxide rich vapour evolved in step (c) thereby producing a carbon dioxide fraction; (e) condensing said carbon dioxide fraction to reform liquid carbon dioxide and recycling said reformed liquid carbon dioxide to contaction step (a); (f) continuing said recycling of the reformed liquid carbon dioxide to increase the concentration of ethanol and so produce a concentrated dry mix ture; (g) feeding said concentrated dry mixture containing ethanol and carbon dioxide to a distillation column having a cooled top and a heated bottom and re covering substantially water free ethanol from said bottom of said distillation column. 2. A method according to claim 1, in which said combined mixture of ethanol and liquid carbon dixoide leaving said contaction step (a) has its ratio of ethanol to water increased to at least 9:1 during said contaction step. 3. A method according to claim 1, in which the con centration of ethanol in said concentrated dry mixture is increased in step (f) until it is present at at least % w/w. 4. A method according to claim 1, in which said liquid ethanol water mixture is subjected to an initial concentration process before said contaction step (a). 5. A method according to claim 1, in which said liquid ethanol water mixture is obtained by a continuous fermentation and primary distillation step in which a continuous fermentation process is employed with a substrate to be fermented being introduced continu ously into a fermenter and a fermented wash resulting from such fermentation being distilled to provide an output liquid ethanol water mixture containing between 30% and 40% ethanol w/w. 6. A method according to claim 1, in which said adsorbent is a crystalline zeolite having a pore aperture size of substantially 3 Angstroms (0.3 nm). 7. A method according to claim 1, in which said liquid ethanol water mixture is produced by fermenta tion and carbon dioxide produced during said fermenta tion is used to provide said liquid carbon dioxide and in which dry substantially pure carbon dioxide is pro duced as an additional product by recovering it as a product from said top of said distillation column in step (g). 8. A method according to claim 6, in which said liquid ethanol water mixture is produced by fermenta tion and carbon dioxide produced during said fermenta tion is used to provide said liquid carbon dioxide and in which dry substantially pure carbon dioxide is pro duced as an additional product by recovering it as a product from said top of said distillation column in step (g). 9. A method according to claim 1, wherein said mix ture containing water and ethanol is produced by fer mentation, and wherein carbon dioxide produced in said fermentation is used as a liquid carbon dioxide in step (a), and wherein said adsorbent which adsorbs substantially all of said water from said ethanol/water/- carbon dioxide mixture is regenerated by heating, and the heat for regeneration of said adsorbent is provided by compressing carbon dioxide produced by fermenta tion to said liquid carbon dioxide for contacting said liquid ethanol/water mixture. 10. A method according to claim 1, in which said substantially water-free ethanol obtained from said bot tom of said distillation column is subjected to a frac tional distillation process to separate ethanol and conge
10 11 ners and provide a substantially pure anhydrous ethanol product. 11. A method of removing water from a mixture containing water and ethanol comprising the steps of: (a) contacting a liquid ethanol water mixture with liquid carbon dioxide as a contaction step whereby ethanol is preferentially transferred from said liq uid ethanol water mixture into solution with said liquid carbon dioxide to increase the ratio of etha nol to water in said liquid carbon dioxide and pro vide a first fraction comprising ethanol/water and a second fraction comprising ethanol/water/car bon dioxide; (b) supplying heat to said second fraction comprising ethanol/water/carbon dioxide to cause volatiliza tion of a fraction rich in carbon dioxide and remov ing said carbon dioxide rich fraction to thereby increase the proportion of ethanol in the remaining fraction; (c) scrubbing the carbon dioxide rich vapour evolved in step (b) to remove substantially all of said etha noi from said carbon dioxide rich vapour evolved in step (b) thereby producing a carbon dioxide fraction; (d) condensing said carbon dioxide fraction to reform liquid carbon dioxide and recycling said reformed liquid carbon dioxide to contaction step (a); (e) continuing said recycling of the reformed liquid carbon dioxide to increase the concentration of 30 ethanol and so produce a concentrated mixture; (f) drying said second fraction comprising ethanol/- water/carbon dioxide resulting from step (e) to produce a concentrated dry mixture comprising ethanol and carbon dioxide by a process including contacting said combined mixture with an adsor bent which adsorbs substantially all of said water from it; (g) feeding said concentrated dry mixture containing ethanol and carbon dioxide to a distillation column having a cooled top and a heated bottom and re covering substantially water free ethanol from said botton of said distillation column. 12. A method according to claim 11 in which said combined mixture of ethanol and liquid carbon dioxide leaving said contaction step (a) has its ratio of ethanol to water increased to at least 9:1 during said contaction step. 13. A method according to claim 11, in which the concentration of ethanol in said concentrated mixture is increased in step (e) until it is present at at least % w/w. 14. A method according to claim 11, in which said liquid ethanol water mixture is subjected to an initial concentration process before said contaction step (a). 15. A method according to claim 11, in which said liquid ethanol water mixture is obtained by a continuous fermentation and primary distillation step in which a continuous fermentation process is employed with a substrate to be fermented being introduced continu ously into a fermenter and a fermented wash resulting from such fermentation being distilled to provide an output liquid ethanol water mixture containing between 30% and 40% ethanol w/w. 16. A method according to claim 11, in which said adsorbent is a crystalline zeolite having a pore aperture size of substantially 3 Angstroms (0.3 nm). 17. A method according to claim 11, in which said liquid ethanol water mixture is produced by fermenta tion and carbon dioxide produced during said fermenta tion is used to provide said liquid carbon dioxide, and in which dry substantially pure carbon dioxide is pro duced as an additional product by recovering it as a product from said top of said distillation column in step (g). 18. A method according to claim 11, in which said liquid ethanol water mixture is produced by fermenta tion and carbon dioxide produced during said fermenta tion is used to provide said liquid carbon dioxide, and in which dry substantially pure carbon dioxide is pro duced as an additional product by recovering it as a product from said top of said distillation column in step (g). 19. A method according to claim 11, wherein said mixture containing water and ethanol is produced by fermentation, and wherein carbon dioxide produced in said fermentation is used as a liquid carbon dioxide in step (a), and wherein said adsorbent which adsorbs substantially all of said water from said ethanol/water/- carbon dioxide mixture is regenerated by heating, and the heat for regeneration of said adsorbent is provided by compressing carbon dioxide produced by fermenta tion to said liquid carbon dioxide for contacting said liquid ethanol/water mixture.. A method according to claim 11, in which said substantially water-free ethanol obtained from said bot tom of said distillation column is subjected to a frac tional distillation process to separate ethanol and conge ners and provide substantially pure anhydrous ethanol product. sk 55 65
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0083132 A1 Maunder et al. US 20140O83132A1 (43) Pub. Date: Mar. 27, 2014 (54) (75) (73) (21) (22) (86) (30) PROCESS FOR LIQUEFACTION
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0083132 A1 Maunder et al. US 20140O83132A1 (43) Pub. Date: Mar. 27, 2014 (54) (75) (73) (21) (22) (86) (30) PROCESS FOR LIQUEFACTION
... O. HT is. suggy. life " "G" 724a, Azaza (W.A. s: D-E-DE-2. Fig March 20, 1973 W. FORG ET AL 3,721,099 NVENTORS 14 X X
 March 20, 1973 W. FORG ET AL 3,721,099 Filed March 24, 1970 FRACTIONAL CONDENSATION OF NATURAL GAS Fig. 1 1 7 -D-E-DE-2 41 suggy 4. Sheets-Sheet l Q a-p DX 14 X X it it 5 it - "G" - 17... O s: 35 - is
March 20, 1973 W. FORG ET AL 3,721,099 Filed March 24, 1970 FRACTIONAL CONDENSATION OF NATURAL GAS Fig. 1 1 7 -D-E-DE-2 41 suggy 4. Sheets-Sheet l Q a-p DX 14 X X it it 5 it - "G" - 17... O s: 35 - is
N 14. United States Patent (19) 15, W. (11) 4,303, Dec. 1, 1981 T COMPRESSOR 5. The present invention relates to a process for providing
 United States Patent (19) Laguilharre et al. 54 MECHANICAL VAPOR RECOMPRESSION EVAPORATORS (75) Inventors: Pierre R. Laguilharre, Enghien les Bains; Jacques J. Ciboit, Paris, both of France 73 Assignee:
United States Patent (19) Laguilharre et al. 54 MECHANICAL VAPOR RECOMPRESSION EVAPORATORS (75) Inventors: Pierre R. Laguilharre, Enghien les Bains; Jacques J. Ciboit, Paris, both of France 73 Assignee:
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O145000A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0145000 A1 Chaubey et al. (43) Pub. Date: Jun. 14, 2012 (54) DRYING PROCESS FOR FLUE GAS (52) U.S. Cl....
(19) United States US 2012O145000A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0145000 A1 Chaubey et al. (43) Pub. Date: Jun. 14, 2012 (54) DRYING PROCESS FOR FLUE GAS (52) U.S. Cl....
WALTER MANN. Jan. 7, 1969 G, SCHAEFER ETA 3,420,750 F. G. FREDRICH WRTH DISTILLING PHTHALIC ANHYDRIDE. Anhydride. Filed May 19, 1967 Sheet / of 2
 Jan. 7, 1969 G, SCHAEFER ETA 3,420,70 DISTILLING PHTHALIC ANHYDRIDE Filed May 19, 1967 Sheet / of 2 F. G. Condenser Condenser Crude Liquid Phtholic Anhydride Feed Falling Film Folling Film NVENTORS: GER
Jan. 7, 1969 G, SCHAEFER ETA 3,420,70 DISTILLING PHTHALIC ANHYDRIDE Filed May 19, 1967 Sheet / of 2 F. G. Condenser Condenser Crude Liquid Phtholic Anhydride Feed Falling Film Folling Film NVENTORS: GER
Dec. 15, ,318. Filed July 26, Sheets-Sheet l REFRIGERATING SYSTEM N. H. GAY
 Dec. 1, 1931. N. H. GAY 1836,318 REFRIGERATING SYSTEM Filed July 26, 1926 2 Sheets-Sheet l Dec. 1, 1931. N. H. GAY REFRIGERATING SYSTEM Filed July 26, l926 l,836,318 2 Sheets-Sheet 2 Patented Dec. 1, 1931
Dec. 1, 1931. N. H. GAY 1836,318 REFRIGERATING SYSTEM Filed July 26, 1926 2 Sheets-Sheet l Dec. 1, 1931. N. H. GAY REFRIGERATING SYSTEM Filed July 26, l926 l,836,318 2 Sheets-Sheet 2 Patented Dec. 1, 1931
(12) United States Patent
 US008011 196B2 (12) United States Patent Eber et al. (54) REFRIGERANT CONTROL OF A HEATRECOVERY CHILLER (75) Inventors: Alan Hv Eber, La Crosse, WI (US); Steven J. Pitts, LaCrescent, MN (US); Brian T.
US008011 196B2 (12) United States Patent Eber et al. (54) REFRIGERANT CONTROL OF A HEATRECOVERY CHILLER (75) Inventors: Alan Hv Eber, La Crosse, WI (US); Steven J. Pitts, LaCrescent, MN (US); Brian T.
United States Patent (19) Cook
 United States Patent (19) Cook (54) SOLAR WATER HEATING SYSTEM (75) Inventor: Robert E. Cook, Kankakee, Ill. 73 Assignee: A. O. Smith Corporation, Milwaukee, Wis. 21 Appl. No.: 708,876 (22 Filed: Jul.
United States Patent (19) Cook (54) SOLAR WATER HEATING SYSTEM (75) Inventor: Robert E. Cook, Kankakee, Ill. 73 Assignee: A. O. Smith Corporation, Milwaukee, Wis. 21 Appl. No.: 708,876 (22 Filed: Jul.
?till SPTT T. United States Patent (19) ea O ----m-m-m-m-m-m- Charpentier et al. 72K7777. ZZZZZZZZ
 United States Patent (19) Charpentier et al. 54 PROCESS AND DEVICE FOR CORRECTING THE OVALIZATION OF ROLLS FOR THE CONTINUOUS CASTING OF METAL STRIP (75 Inventors: Jacques Charpentier, Saint Julien de
United States Patent (19) Charpentier et al. 54 PROCESS AND DEVICE FOR CORRECTING THE OVALIZATION OF ROLLS FOR THE CONTINUOUS CASTING OF METAL STRIP (75 Inventors: Jacques Charpentier, Saint Julien de
(12) United States Patent
 (12) United States Patent Kuroki et al. USOO6467288B2 (10) Patent No.: (45) Date of Patent: Oct. 22, 2002 (54) HEAT-PUMP WATER HEATER (75) Inventors: Jyouji Kuroki, Kariya (JP); Hisayoshi Sakakibara, Nishio
(12) United States Patent Kuroki et al. USOO6467288B2 (10) Patent No.: (45) Date of Patent: Oct. 22, 2002 (54) HEAT-PUMP WATER HEATER (75) Inventors: Jyouji Kuroki, Kariya (JP); Hisayoshi Sakakibara, Nishio
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States US 20040206110A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0206110 A1 Lifson et al. (43) Pub. Date: (54) VAPOR COMPRESSION SYSTEM WITH BYPASS/ECONOMIZER CIRCUITS (76)
(19) United States US 20040206110A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0206110 A1 Lifson et al. (43) Pub. Date: (54) VAPOR COMPRESSION SYSTEM WITH BYPASS/ECONOMIZER CIRCUITS (76)
United States Patent (19) Bratt
 United States Patent (19) Bratt 54) (75) (73) 21 22 63) (51) (52) (58) (56) HOT GAS ENGINE HEATER HEAD Inventor: Jan C. Bratt, Malmö, Sweden Assignee: United Stirling AB, Malmö, Sweden Appl. No.: 852,071
United States Patent (19) Bratt 54) (75) (73) 21 22 63) (51) (52) (58) (56) HOT GAS ENGINE HEATER HEAD Inventor: Jan C. Bratt, Malmö, Sweden Assignee: United Stirling AB, Malmö, Sweden Appl. No.: 852,071
United States Patent (19) Buck et al.
 United States Patent (19) Buck et al. 11 Patent Number: 45) Date of Patent: 4,895,584 Jan. 23, 1990 (54) PROCESS FOR CRECOVERY 75 Inventors: Loren L. Buck, Tulsa; Ronald D. Key, Broken Arrow, both of Okla.
United States Patent (19) Buck et al. 11 Patent Number: 45) Date of Patent: 4,895,584 Jan. 23, 1990 (54) PROCESS FOR CRECOVERY 75 Inventors: Loren L. Buck, Tulsa; Ronald D. Key, Broken Arrow, both of Okla.
9-rea 7 C %.11a- 27, Célé. Sept. 2, ,507,108. PROCESS AND APPARATUS FOR THE CONTINUOUS DISTILLATION OF ALCOHOL Filed Sept. 21.
 Sept. 2, 1924. 1,07,108 s J. F. CYPHERS PRCESS AND APPARATUS FR THE CNTINUUS DISTILLATIN F ALCHL Filed Sept. 21. 1923 'ili ill fill INVENTR. 9-rea 7 C-464 2%.11a- 27, Célé ATTRNEY. 20 4 0 Patented Sept.
Sept. 2, 1924. 1,07,108 s J. F. CYPHERS PRCESS AND APPARATUS FR THE CNTINUUS DISTILLATIN F ALCHL Filed Sept. 21. 1923 'ili ill fill INVENTR. 9-rea 7 C-464 2%.11a- 27, Célé ATTRNEY. 20 4 0 Patented Sept.
CD?inge ) 34 48
 USOO9435040B2 (12) United States Patent Hasegawa et al. () Patent No.: (45) Date of Patent: US 9.435,040 B2 Sep. 6, 2016 (54) (71) WATER ELECTROLYSIS SYSTEM Applicant: NISSAN MOTOR CO., LTD., Yokohama-shi,
USOO9435040B2 (12) United States Patent Hasegawa et al. () Patent No.: (45) Date of Patent: US 9.435,040 B2 Sep. 6, 2016 (54) (71) WATER ELECTROLYSIS SYSTEM Applicant: NISSAN MOTOR CO., LTD., Yokohama-shi,
United States Patent (19) Paradowski et al.
 United States Patent (19) Paradowski et al. 11) Patent Number: Date of Patent: Aug., 1987 54 PROCESS OF FRACTIONATING GAS FEEDS AND APPARATUS FOR CARRYING OUT THE SAD PROCESS (75) Inventors: Henri Paradowski,
United States Patent (19) Paradowski et al. 11) Patent Number: Date of Patent: Aug., 1987 54 PROCESS OF FRACTIONATING GAS FEEDS AND APPARATUS FOR CARRYING OUT THE SAD PROCESS (75) Inventors: Henri Paradowski,
United States Patent (19) Henle
 United States Patent (19) Henle 54 COMBINATION WATER SUPPLY AND WASTE HOLDING TANK (76) Inventor: George A. Henle, 920 Penfield St., Beecher, Ill. 60401 22 Filed: Jan. 15, 1975 (21) Appl. No.: 541,225
United States Patent (19) Henle 54 COMBINATION WATER SUPPLY AND WASTE HOLDING TANK (76) Inventor: George A. Henle, 920 Penfield St., Beecher, Ill. 60401 22 Filed: Jan. 15, 1975 (21) Appl. No.: 541,225
US A United States Patent (19) 11 Patent Number: 6,092,490 Bairley et al. (45) Date of Patent: Jul. 25, 2000
 US0060924.90A United States Patent (19) 11 Patent Number: 6,092,490 Bairley et al. (45) Date of Patent: Jul. 25, 2000 54) HEAT RECVERY STEAM GENERATR 4,858,562 8/1989 Arakawa et al.... 122/7 R 5,159,897
US0060924.90A United States Patent (19) 11 Patent Number: 6,092,490 Bairley et al. (45) Date of Patent: Jul. 25, 2000 54) HEAT RECVERY STEAM GENERATR 4,858,562 8/1989 Arakawa et al.... 122/7 R 5,159,897
United States Patent (19) Koskela
 United States Patent (19) Koskela 54 (76) (21) 22) 51 52) (58) 56 SOLAR WATER HEATING SYSTEMAND HEAT EXCHANGER FOR USE WITH EXISTING HOT WATER SYSTEMS Inventor: Marvin O. Koskela, 4222 E. Calle Redonda,
United States Patent (19) Koskela 54 (76) (21) 22) 51 52) (58) 56 SOLAR WATER HEATING SYSTEMAND HEAT EXCHANGER FOR USE WITH EXISTING HOT WATER SYSTEMS Inventor: Marvin O. Koskela, 4222 E. Calle Redonda,
(12) (10) Patent No.: US 7, B2 Army, Jr. et al. (45) Date of Patent: Mar. 13, 2007
 United States Patent USOO7188488B2 (12) (10) Patent No.: Army, Jr. et al. (45) Date of Patent: Mar. 13, 2007 (54) PACK AND A HALF CONDENSING CYCLE 2003/0084681 A1* 5/2003 Haas... 62/402 PACK WITH COMBINED
United States Patent USOO7188488B2 (12) (10) Patent No.: Army, Jr. et al. (45) Date of Patent: Mar. 13, 2007 (54) PACK AND A HALF CONDENSING CYCLE 2003/0084681 A1* 5/2003 Haas... 62/402 PACK WITH COMBINED
(12) United States Patent (10) Patent No.: US 7,014,690 B2
 USOO7014690B2 (12) United States Patent (10) Patent No.: US 7,014,690 B2 Mitsch et al. (45) Date of Patent: Mar. 21, 2006 (54) EXPANDABLE DESICCANTELEMENT 5,689,893 A 11/1997 Mitsch 5,715,621. A 2/1998
USOO7014690B2 (12) United States Patent (10) Patent No.: US 7,014,690 B2 Mitsch et al. (45) Date of Patent: Mar. 21, 2006 (54) EXPANDABLE DESICCANTELEMENT 5,689,893 A 11/1997 Mitsch 5,715,621. A 2/1998
(12) United States Patent (10) Patent No.: US 6,560,989 B1
 USOO989B1 (12) United States Patent (10) Patent No.: Roberts et al. () Date of Patent: May 13, 2003 (54) SEPARATION OF 5,333,462 A 8/1994 Garot et al.... 62/24 HYDROGEN-HYDROCARBON GAS 5,414,168 A 5/1995
USOO989B1 (12) United States Patent (10) Patent No.: Roberts et al. () Date of Patent: May 13, 2003 (54) SEPARATION OF 5,333,462 A 8/1994 Garot et al.... 62/24 HYDROGEN-HYDROCARBON GAS 5,414,168 A 5/1995
(12) United States Patent (10) Patent No.: US 6,176,097 B1. Kim (45) Date of Patent: Jan. 23, 2001
 USOO6176097B1 (12) United States Patent (10) Patent No.: Kim (45) Date of Patent: Jan. 23, 2001 (54) SIDE BY SIDE TYPE REFRIGERATOR AND 5,477,699 12/1995 Guess et al.... 62/187 METHOD FOR CONTROLLING 5,732,561
USOO6176097B1 (12) United States Patent (10) Patent No.: Kim (45) Date of Patent: Jan. 23, 2001 (54) SIDE BY SIDE TYPE REFRIGERATOR AND 5,477,699 12/1995 Guess et al.... 62/187 METHOD FOR CONTROLLING 5,732,561
US A United States Patent (19) 11) Patent Number: 5,573,058 Rolin (45) Date of Patent: Nov. 12, Sweden B /1981 Finland.
 US005573058A United States Patent (19) 11) Patent Number: Rolin (45) Date of Patent: Nov. 12, 1996 54 AIR-CONDITIONING INSTALLATION FOR 4,084,635 4/1978 Marshall... 165/909 ROOM SPACES 4,142,575 3/1979
US005573058A United States Patent (19) 11) Patent Number: Rolin (45) Date of Patent: Nov. 12, 1996 54 AIR-CONDITIONING INSTALLATION FOR 4,084,635 4/1978 Marshall... 165/909 ROOM SPACES 4,142,575 3/1979
United States Patent (19)
 United States Patent (19) Studinger (54) MOBILE SELF CONTAINED PRESSURE SPRAYER 76 Inventor: John H. Studinger, 5700 Montview Blvd., Denver, Colo. 80207 (22 Filed: June 26, 1972 21 Appl. No. 266,415 52
United States Patent (19) Studinger (54) MOBILE SELF CONTAINED PRESSURE SPRAYER 76 Inventor: John H. Studinger, 5700 Montview Blvd., Denver, Colo. 80207 (22 Filed: June 26, 1972 21 Appl. No. 266,415 52
United States Patent (19) Lott
 United States Patent (19) Lott (54) (75) (73) 21 22) (51) (52) (58) 56) APPARATUS FOR ENHANCING THE PERFORMANCE OF A VEHICLE AIR CONDITIONING SYSTEM Inventor: John Lott, Auburndale, Fla. Assignee. Judy
United States Patent (19) Lott (54) (75) (73) 21 22) (51) (52) (58) 56) APPARATUS FOR ENHANCING THE PERFORMANCE OF A VEHICLE AIR CONDITIONING SYSTEM Inventor: John Lott, Auburndale, Fla. Assignee. Judy
San Francisco, Calif (21) Appl. No.: 810, Filed: Jun. 27, Int. Cl... B01F3/04 52 U.S. C /119 R; 55/244;
 United States Patent (19) Genessi (54) LINT INTERCEPTOR 76 Inventor: Richard J. Genessi, 2434 Rivera St., San Francisco, Calif. 941 16 (21) Appl. No.: 810,387 22 Filed: Jun. 27, 1977 51 Int. Cl... B01F3/04
United States Patent (19) Genessi (54) LINT INTERCEPTOR 76 Inventor: Richard J. Genessi, 2434 Rivera St., San Francisco, Calif. 941 16 (21) Appl. No.: 810,387 22 Filed: Jun. 27, 1977 51 Int. Cl... B01F3/04
(12) United States Patent
 USO080427B2 (12) United States Patent Schwartz et al. (54) HYDROGEN LIQUEFACTION METHOD AND LIQUEFIER (75) Inventors: Joseph Michael Schwartz, Williamsville, NY (US); Raymond Francis Drnevich, Clarence
USO080427B2 (12) United States Patent Schwartz et al. (54) HYDROGEN LIQUEFACTION METHOD AND LIQUEFIER (75) Inventors: Joseph Michael Schwartz, Williamsville, NY (US); Raymond Francis Drnevich, Clarence
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 20120312161A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0312161 A1 Reitzle et al. (43) Pub. Date: (54) METHOD AND DEVICE FOR REDUCING (30) Foreign Application Priority
(19) United States US 20120312161A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0312161 A1 Reitzle et al. (43) Pub. Date: (54) METHOD AND DEVICE FOR REDUCING (30) Foreign Application Priority
4-26. United States Patent (19) Woollenweber et al. R XI N Patent Number: 6,102,672 (45) Date of Patent: Aug. 15, (75)
 United States Patent (19) Woollenweber et al. 54 (75) MOTOR-DRIVEN CENTRIFUGAL AIR COMPRESSOR WITH INTERNAL COOLING ARFLOW Inventors: William E. Woollenweber, Carlsbad; Edward M. Halimi, Montecito, both
United States Patent (19) Woollenweber et al. 54 (75) MOTOR-DRIVEN CENTRIFUGAL AIR COMPRESSOR WITH INTERNAL COOLING ARFLOW Inventors: William E. Woollenweber, Carlsbad; Edward M. Halimi, Montecito, both
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 US 2011 0120094A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0120094A1 Crawley et al. (43) Pub. Date: May 26, 2011 (54) METHOD OF REGENERATING AN EXHAUST (30) Foreign
US 2011 0120094A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0120094A1 Crawley et al. (43) Pub. Date: May 26, 2011 (54) METHOD OF REGENERATING AN EXHAUST (30) Foreign
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014O137590A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0137590 A1 Chopko et al. (43) Pub. Date: May 22, 2014 (54) INTEGRATED TRANSPORT Publication Classification
(19) United States US 2014O137590A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0137590 A1 Chopko et al. (43) Pub. Date: May 22, 2014 (54) INTEGRATED TRANSPORT Publication Classification
-i. United States Patent (11) 3,633,37. 6/1931 Davenport... 62/474X. (72 Inventor Lester K. Quick
 United States Patent (72 Inventor Lester K. Quick 868 Westview Crescent, North Vancouver, B. C., Canada 21 Appi. No. 8,2 (22 Filed Apr. 11, 1969 ) Patented Jan. 11, 1972 54 REFRIGERATION SYSTEM OIL SEPARATOR
United States Patent (72 Inventor Lester K. Quick 868 Westview Crescent, North Vancouver, B. C., Canada 21 Appi. No. 8,2 (22 Filed Apr. 11, 1969 ) Patented Jan. 11, 1972 54 REFRIGERATION SYSTEM OIL SEPARATOR
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0017627 A1 Jeong et al. US 201200 17627A1 (43) Pub. Date: Jan. 26, 2012 (54) (75) (73) (21) (22) (86) (30) APPARATUS FOR PURIFYING
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0017627 A1 Jeong et al. US 201200 17627A1 (43) Pub. Date: Jan. 26, 2012 (54) (75) (73) (21) (22) (86) (30) APPARATUS FOR PURIFYING
United States Patent (19) 11 Patent Number: 5,651,270 Low et al. 45 Date of Patent: Jul. 29, 1997
 US005651270A United States Patent (19) 11 Patent Number: Low et al. 45 Date of Patent: Jul. 29, 1997 54 CORE-IN-SHELL HEAT EXCHANGERS FOR Attorney, Agent, or Firm--George E. Bogatie MULTISTAGE COMPRESSORS
US005651270A United States Patent (19) 11 Patent Number: Low et al. 45 Date of Patent: Jul. 29, 1997 54 CORE-IN-SHELL HEAT EXCHANGERS FOR Attorney, Agent, or Firm--George E. Bogatie MULTISTAGE COMPRESSORS
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 (19) United States US 200700.44517A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0044517 A1 Yang et al. (43) Pub. Date: Mar. 1, 2007 (54) DETERGENT SUPPLYING APPARATUS OF CLOTHES WASHING
(19) United States US 200700.44517A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0044517 A1 Yang et al. (43) Pub. Date: Mar. 1, 2007 (54) DETERGENT SUPPLYING APPARATUS OF CLOTHES WASHING
A1(t1) (12) Patent Application Publication (10) Pub. No.: US 2011/ A1. (19) United States. Jiang et al. (43) Pub. Date: Sep.
 (19) United States US 2011 O232884A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0232884 A1 Jiang et al. (43) Pub. Date: Sep. 29, 2011 (54) HEAT EXCHANGER (75) Inventors: Jianlong Jiang,
(19) United States US 2011 O232884A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0232884 A1 Jiang et al. (43) Pub. Date: Sep. 29, 2011 (54) HEAT EXCHANGER (75) Inventors: Jianlong Jiang,
United States Patent (19) 11 3,956,072 Huse (45) May 11, 1976
 United States Patent (19) 11 Huse (45) May 11, 1976 54) VAPOR DISTILLATION APPARATUS WITH 56 References Cited TWO DISPARATE COMPRESSORS UNITED STATES PATENTS (75 Inventor: Henry Huse, Darien, Conn. 849,579
United States Patent (19) 11 Huse (45) May 11, 1976 54) VAPOR DISTILLATION APPARATUS WITH 56 References Cited TWO DISPARATE COMPRESSORS UNITED STATES PATENTS (75 Inventor: Henry Huse, Darien, Conn. 849,579
USOO A United States Patent (19) 11 Patent Number: 5,993,656 Cordani (45) Date of Patent: Nov.30, 1999
 USOO5993656A United States Patent (19) 11 Patent Number: 5,993,656 Cordani (45) Date of Patent: Nov.30, 1999 54). SELECTIVE FLUIDABSORBING DEVICE 4,861,469 8/1989 Rossi et al.... 21.0/502.1 5,130,018 7/1992
USOO5993656A United States Patent (19) 11 Patent Number: 5,993,656 Cordani (45) Date of Patent: Nov.30, 1999 54). SELECTIVE FLUIDABSORBING DEVICE 4,861,469 8/1989 Rossi et al.... 21.0/502.1 5,130,018 7/1992
Jan. 13, ,489,652. DISTILLATION COMBINED WITH POWER GENERATION 3. Sheets-Sheet. Filed April 18, Af777/46/C/ :42, TT /
 Jan. 13, 1970 Filed April 18, 1966 W. R. W.L.AMSON DESALINATION PROCESS BY MULTI-EFFECT, MULTI - STAGE FLASH DISTILLATION COMBINED WITH POWER GENERATION 3. Sheets-Sheet 232 7 Af777/46/C/ :42, TT672757-/
Jan. 13, 1970 Filed April 18, 1966 W. R. W.L.AMSON DESALINATION PROCESS BY MULTI-EFFECT, MULTI - STAGE FLASH DISTILLATION COMBINED WITH POWER GENERATION 3. Sheets-Sheet 232 7 Af777/46/C/ :42, TT672757-/
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1. Weng et al. (43) Pub. Date: Jun. 23, 2005
 (19) United States US 2005O133195A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0133195A1 Weng et al. (43) Pub. Date: Jun. 23, 2005 (54) HEAT EXCHANGER USING WATER LIQUID (52) U.S. C.. 165/53
(19) United States US 2005O133195A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0133195A1 Weng et al. (43) Pub. Date: Jun. 23, 2005 (54) HEAT EXCHANGER USING WATER LIQUID (52) U.S. C.. 165/53
????% dt???5. 3,351,120. Nov. 7, 1967 R. W. GOELDNER ET AL MULTIPLE EFFECT, MULTI-STAGE FLASH AND FILM EWAPORATOR NVENTORS RICHARD W.
 Nov. 7, 1967 3,31,1 R. W. GOELDNER ET AL MULTIPLE EFFECT, MULTI-STAGE FLASH AND FILM EWAPORATOR NVENTORS RICHARD W. GOELDNER BY ARMANDO E3 STEINBARUCHEL????% dt???. Nov. 7, 1967 R. W. GOELDNER ET AL 3
Nov. 7, 1967 3,31,1 R. W. GOELDNER ET AL MULTIPLE EFFECT, MULTI-STAGE FLASH AND FILM EWAPORATOR NVENTORS RICHARD W. GOELDNER BY ARMANDO E3 STEINBARUCHEL????% dt???. Nov. 7, 1967 R. W. GOELDNER ET AL 3
(2) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United tates U 20090094991A1 (2) Patent Application Publication (10) Pub. No.: U 2009/0094991A1 Yu et al. (43) Pub. Date: Apr. 16, 2009 9 (54) HIGH EFFICIENCY HYBRID AIR Publication Classification
(19) United tates U 20090094991A1 (2) Patent Application Publication (10) Pub. No.: U 2009/0094991A1 Yu et al. (43) Pub. Date: Apr. 16, 2009 9 (54) HIGH EFFICIENCY HYBRID AIR Publication Classification
United States Patent (19) Endo et al.
 United States Patent (19) Endo et al. 11 Patent Number: (45) Date of Patent: 4,656,334 Apr. 7, 1987 (54) BED WARMER WITH ABODY TEMPERATURE SENSOR FOR STOPPINGA HIGHER PRESET TEMPERATURE 75) Inventors:
United States Patent (19) Endo et al. 11 Patent Number: (45) Date of Patent: 4,656,334 Apr. 7, 1987 (54) BED WARMER WITH ABODY TEMPERATURE SENSOR FOR STOPPINGA HIGHER PRESET TEMPERATURE 75) Inventors:
SYS; Só-N III. sžess 43. United States Patent (19) Voorhis 5,706, Jan. 13, Date of Patent: Patent Number:
 United States Patent (19) Voorhis III 11 45 US005706670A Patent Number: Date of Patent: Jan. 13, 1998 54 BDIRECTIONAL METERD FLOW CONTROL DEVICE (75) 73 21 22 51 52 58) 56 Inventor: Roger J. Voorhis, Pennellville,
United States Patent (19) Voorhis III 11 45 US005706670A Patent Number: Date of Patent: Jan. 13, 1998 54 BDIRECTIONAL METERD FLOW CONTROL DEVICE (75) 73 21 22 51 52 58) 56 Inventor: Roger J. Voorhis, Pennellville,
United States Patent (19) More
 United States Patent (19) More 11 Patent Number: 45 Date of Patent: Nov. 27, 1984 54 SHREDDING MACHINE FOR RECYCLING TEXT LE FIBERS AND METHOD (75) Inventor: André Morel, La Croix du Mont, France (73)
United States Patent (19) More 11 Patent Number: 45 Date of Patent: Nov. 27, 1984 54 SHREDDING MACHINE FOR RECYCLING TEXT LE FIBERS AND METHOD (75) Inventor: André Morel, La Croix du Mont, France (73)
United States Patent (19) Seidel et al.
 United States Patent (19) Seidel et al. 54 SOLAR-THERMAL POWER PLANT 75) Inventors: Albert Seidel; Dietmar Wolf, both of Siegertsbrunn, Fed. Rep. of Germany 73) Assignee: Messerschmitt-Bölkow Blohm Gesellschaft
United States Patent (19) Seidel et al. 54 SOLAR-THERMAL POWER PLANT 75) Inventors: Albert Seidel; Dietmar Wolf, both of Siegertsbrunn, Fed. Rep. of Germany 73) Assignee: Messerschmitt-Bölkow Blohm Gesellschaft
219,432,433,436,528,529, 99,483 is ABSTRACT 56) References Cited
 USOO6075229A United States Patent (19) 11 Patent Number: 6,075,229 Vanselow (45) Date of Patent: Jun. 13, 2000 54). CUP WARMER HOLDER 4,442,343 4/1984 Genuit et al.... 219/433 4,463,664 8/1984 Peace......
USOO6075229A United States Patent (19) 11 Patent Number: 6,075,229 Vanselow (45) Date of Patent: Jun. 13, 2000 54). CUP WARMER HOLDER 4,442,343 4/1984 Genuit et al.... 219/433 4,463,664 8/1984 Peace......
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 2007.0056318A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0056318 A1 Ransbarger (43) Pub. Date: (54) ENHANCED HEAVIES REMOVAL/LPG Publication Classification RECOVERY
US 2007.0056318A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0056318 A1 Ransbarger (43) Pub. Date: (54) ENHANCED HEAVIES REMOVAL/LPG Publication Classification RECOVERY
(12) United States Patent (10) Patent No.: US 6,920,917 B2
 USOO6920917B2 (12) United States Patent (10) Patent No.: Inoue et al. (45) Date of Patent: Jul. 26, 2005 (54) DOUBLE-PIPE HEAT EXCHANGER 5,950,716 A 9/1999 Appelquist et al.... 165/109.1 6,220,344 B1 *
USOO6920917B2 (12) United States Patent (10) Patent No.: Inoue et al. (45) Date of Patent: Jul. 26, 2005 (54) DOUBLE-PIPE HEAT EXCHANGER 5,950,716 A 9/1999 Appelquist et al.... 165/109.1 6,220,344 B1 *
United States Patent (19) Olin et al.
 United States Patent (19) Olin et al. 54) VACUUM TOILET UNIT 75 Inventors: Henry Olin, Espoo; Gunner Lindroos, Helsinki; Roland Mattsson, Espoo, all of Finland 73 Assignee: Evac International Oy, Helsinki,
United States Patent (19) Olin et al. 54) VACUUM TOILET UNIT 75 Inventors: Henry Olin, Espoo; Gunner Lindroos, Helsinki; Roland Mattsson, Espoo, all of Finland 73 Assignee: Evac International Oy, Helsinki,
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 (19) United States US 20070209656A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0209656A1 Lee (43) Pub. Date: Sep. 13, 2007 (54) VAPOR HEATING TYPE COOKING APPARATUS (76) Inventor: Won-Ki
(19) United States US 20070209656A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0209656A1 Lee (43) Pub. Date: Sep. 13, 2007 (54) VAPOR HEATING TYPE COOKING APPARATUS (76) Inventor: Won-Ki
MD-2, D -2 (5-37-se Gas
 US006053007A United States Patent (19) 11 Patent Number: Victory et al. (45) Date of Patent: Apr. 25, 2000 54 PROCESS FOR SEPARATING A 5,062,270 11/1991 Haut et al.... 62/620 MULTI-COMPONENT GAS STREAM
US006053007A United States Patent (19) 11 Patent Number: Victory et al. (45) Date of Patent: Apr. 25, 2000 54 PROCESS FOR SEPARATING A 5,062,270 11/1991 Haut et al.... 62/620 MULTI-COMPONENT GAS STREAM
Tikhonov et al. (45) Date of Patent: Mar. 13, (54) REFRIGERATOR WITH SELECTIVE (56) References Cited ARFLOWPASSAGES BETWEEN THE
 (12) United States Patent USOO8132423B2 () Patent No.: US 8,132,423 B2 Tikhonov et al. (45) Date of Patent: Mar. 13, 2012 (54) REFRIGERATOR WITH SELECTIVE (56) References Cited ARFLOWPASSAGES BETWEEN THE
(12) United States Patent USOO8132423B2 () Patent No.: US 8,132,423 B2 Tikhonov et al. (45) Date of Patent: Mar. 13, 2012 (54) REFRIGERATOR WITH SELECTIVE (56) References Cited ARFLOWPASSAGES BETWEEN THE
United States Patent (19) 11 Patent Number: 4,682,610 Freelain 45 Date of Patent: Jul. 28, 1987
 United States Patent (19) 11 Patent Number: Freelain 45 Date of Patent: Jul. 28, 1987 54, WATER PIPE 23.9 5/1980 Howell, Jr.... E.,215,707 8/1980 Elrich... 31/17 76 Inventor: Kenneth W. Freelain, 1630-A
United States Patent (19) 11 Patent Number: Freelain 45 Date of Patent: Jul. 28, 1987 54, WATER PIPE 23.9 5/1980 Howell, Jr.... E.,215,707 8/1980 Elrich... 31/17 76 Inventor: Kenneth W. Freelain, 1630-A
United States Patent Modine et al.
 United States Patent Modine et al. 54 MODULAR AR COOLED CONDENSER 72) Inventors: Arthur B. Modine; Homer D. Hug gins; Neal A. Cook, all of Racine, Wis. 73) Assignee: Modine Manufacturing Company 22 Filed:
United States Patent Modine et al. 54 MODULAR AR COOLED CONDENSER 72) Inventors: Arthur B. Modine; Homer D. Hug gins; Neal A. Cook, all of Racine, Wis. 73) Assignee: Modine Manufacturing Company 22 Filed:
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005.0072175A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0072175A1 Umeo et al. (43) Pub. Date: Apr. 7, 2005 (54) AIR CONDITIONER ANDTRUCK EQUIPPED WITH SAME (76)
(19) United States US 2005.0072175A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0072175A1 Umeo et al. (43) Pub. Date: Apr. 7, 2005 (54) AIR CONDITIONER ANDTRUCK EQUIPPED WITH SAME (76)
52 U.S. C... 62/ Field of Search... 62/256 56) References Cited U.S. PATENT DOCUMENTS 4,312,190 1/1982 Ibrahim et al...
 United States Patent (19) Tanaka 11 Patent Number: 45 Date of Patent: Oct. 23, 1990 (54) LOW-TEMPERATURE SHOWCASE 75) Inventor: Tsutomu Tanaka, Oizumi, Japan 73) Assignee: Sanyo Electric Co., Ltd., Osaka,
United States Patent (19) Tanaka 11 Patent Number: 45 Date of Patent: Oct. 23, 1990 (54) LOW-TEMPERATURE SHOWCASE 75) Inventor: Tsutomu Tanaka, Oizumi, Japan 73) Assignee: Sanyo Electric Co., Ltd., Osaka,
United States Patent (19) Koskela
 United States Patent (19) Koskela 54 SOLAR WATER HEATING SYSTEM AND HEAT EXCHANGER THEREFOR 76 Inventor: Marvin O. Koskela, 4222 E. Calle Redondo, Phoenix, Ariz. 818 21 Appl. No.: 106,539 22) Filed: Dec.
United States Patent (19) Koskela 54 SOLAR WATER HEATING SYSTEM AND HEAT EXCHANGER THEREFOR 76 Inventor: Marvin O. Koskela, 4222 E. Calle Redondo, Phoenix, Ariz. 818 21 Appl. No.: 106,539 22) Filed: Dec.
United States Patent (19) Decker
 United States Patent (19) Decker 54 GANTRY-TYPE WASHING INSTALLATION FOR WASHING MOTOR VEHICLE 75 Inventor: Wolfgang Decker, Zusmarshausen-Wollbach, Germany 73 Assignee: Wesumat Fahrzeugwaschanlagen GmbH,
United States Patent (19) Decker 54 GANTRY-TYPE WASHING INSTALLATION FOR WASHING MOTOR VEHICLE 75 Inventor: Wolfgang Decker, Zusmarshausen-Wollbach, Germany 73 Assignee: Wesumat Fahrzeugwaschanlagen GmbH,
United States Patent (19) Dean
 United States Patent (19) Dean 54 (76) 21) 22 63 51 52 58) 56) ARVENTTLATION CONTROL SYSTEM Inventor: Arthur C. Dean, 13403 Vimy Ridge Rd., Alexander, Ark. 72002 Appl. No.: 63,429 Filed: Jun. 18, 1987
United States Patent (19) Dean 54 (76) 21) 22 63 51 52 58) 56) ARVENTTLATION CONTROL SYSTEM Inventor: Arthur C. Dean, 13403 Vimy Ridge Rd., Alexander, Ark. 72002 Appl. No.: 63,429 Filed: Jun. 18, 1987
-50. Liquid outlet 1-1. Liquid outlet 2-1. Liquid outlet b. Liquid outlet 4-1. N-Liquid inlet 4. N-Liquid inlet 2.
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0196442 A1 Lu US 2008O196442A1 (43) Pub. Date: Aug. 21, 2008 (54) (75) (73) (21) (22) (60) AIRCRAFT GALLEY REFRGERATION SYSTEM
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0196442 A1 Lu US 2008O196442A1 (43) Pub. Date: Aug. 21, 2008 (54) (75) (73) (21) (22) (60) AIRCRAFT GALLEY REFRGERATION SYSTEM
(12) United States Patent
 (12) United States Patent USOO7356873B2 (10) Patent No.: US 7,356,873 B2 Nielsen (45) Date of Patent: Apr. 15, 2008 (54) HIGHLY EFFICIENT AUTONOMOUS 3,592,566 A 7, 1971 Beardslee VACUUM CLEANER 3,906,585
(12) United States Patent USOO7356873B2 (10) Patent No.: US 7,356,873 B2 Nielsen (45) Date of Patent: Apr. 15, 2008 (54) HIGHLY EFFICIENT AUTONOMOUS 3,592,566 A 7, 1971 Beardslee VACUUM CLEANER 3,906,585
Oct. 11, M. E. PENNINGTON 1,882,030 CONDITIONING SYSTEM FOR COLD STORAGE ROOMS
 Oct. 11, 1932. M. E. PENNINGTON 1,882,030 CONDITIONING SYSTEM FOR COLD STORAGE ROOMS 24%23. Filed Oct. 18, 1929 a 2. s 2 % 2 2 s2 2 % 2 as sease teams ass=sessessessessessesserences 2 2 272,222 % % 2.
Oct. 11, 1932. M. E. PENNINGTON 1,882,030 CONDITIONING SYSTEM FOR COLD STORAGE ROOMS 24%23. Filed Oct. 18, 1929 a 2. s 2 % 2 2 s2 2 % 2 as sease teams ass=sessessessessessesserences 2 2 272,222 % % 2.
(%rané- a vectrar F. G. HEAT OUT HEAT /W F. G. 2 COOLING APPARATUS AND PROCESS. Nov. 23, 1971 R. G. MOKADAM 3,621,667 EVAPORATION
 Nov. 23, 1971 R. G. MOKADAM COOLING APPARATUS AND PROCESS Filed Nov. 28, 1969 2 Sheets-Sheet F. G. HEAT OUT HEAT /W F. G. 2 ARESSURIZED COWD/7/OW COWDEWSATION COWD/7/O/W EVAPORATION COWD/7/O/W EWTHALPY
Nov. 23, 1971 R. G. MOKADAM COOLING APPARATUS AND PROCESS Filed Nov. 28, 1969 2 Sheets-Sheet F. G. HEAT OUT HEAT /W F. G. 2 ARESSURIZED COWD/7/OW COWDEWSATION COWD/7/O/W EVAPORATION COWD/7/O/W EWTHALPY
Arrangements of cold exchangers or cold accumulators in cryogenic separation or liquefaction plants.
 CPC - F25J - 2017.08 F25J LIQUEFACTION, SOLIDIFICATION OR SEPARATION OF GASES OR GASEOUS {OR LIQUEFIED GASEOUS} MIXTURES BY PRESSURE AND COLD TREATMENT {OR BY BRINGING THEM INTO THE SUPERCRITICAL STATE
CPC - F25J - 2017.08 F25J LIQUEFACTION, SOLIDIFICATION OR SEPARATION OF GASES OR GASEOUS {OR LIQUEFIED GASEOUS} MIXTURES BY PRESSURE AND COLD TREATMENT {OR BY BRINGING THEM INTO THE SUPERCRITICAL STATE
(12) United States Patent (10) Patent No.: US 6,257,007 B1
 USOO6257007B1 (12) United States Patent (10) Patent No.: US 6,257,007 B1 Hartman (45) Date of Patent: Jul. 10, 2001 (54) METHOD OF CONTROL OF COOLING 6,065,298 * 5/2000 Fujimoto... 62/230 SYSTEM CONDENSER
USOO6257007B1 (12) United States Patent (10) Patent No.: US 6,257,007 B1 Hartman (45) Date of Patent: Jul. 10, 2001 (54) METHOD OF CONTROL OF COOLING 6,065,298 * 5/2000 Fujimoto... 62/230 SYSTEM CONDENSER
l SS se S III United States Patent (19) Klobucar (21) Appl. No.: Filed: Jul. 2, Claims, 4 Drawing Sheets
 United States Patent (19) Klobucar 54 REGENERATIVE THERMAL OXDZER WITH HEAT EXCHANGER COLUMNS 75 Inventor: Joseph M. Klobucar, Plymouth, Mich. 73 Assignee: Durr Industries, Inc., Plymouth, Mich. (21) Appl.
United States Patent (19) Klobucar 54 REGENERATIVE THERMAL OXDZER WITH HEAT EXCHANGER COLUMNS 75 Inventor: Joseph M. Klobucar, Plymouth, Mich. 73 Assignee: Durr Industries, Inc., Plymouth, Mich. (21) Appl.
United States Patent (19) (11) Patent Number: 5,033,657
 United States Patent (19) (11) Patent Number: 5,033,657 Whittington 45) Date of Patent: Jul. 23, 1991 54 ADJUSTABLESTROKE PUMP DISPENSER 4,978,036 12/1990 Burd... 222/2O7 75) Inventor: Jimmie L. Whittington,
United States Patent (19) (11) Patent Number: 5,033,657 Whittington 45) Date of Patent: Jul. 23, 1991 54 ADJUSTABLESTROKE PUMP DISPENSER 4,978,036 12/1990 Burd... 222/2O7 75) Inventor: Jimmie L. Whittington,
ACD /. United States Patent (19) Standiford (1) 3,968,002. (45) July 6, 1976 (54) FEED HEATING METHOD FOR MULTIPLE
 United States Patent (19) Standiford (4) FEED HEATING METHOD FOR MULTIPLE EFFECT EVAPORATORS 76) inventor: Ferris C. Standiford, 2713 S. North Bluff Road, Greenbank, Wash. 9823 22 Filed: Mar. 7, 197 21
United States Patent (19) Standiford (4) FEED HEATING METHOD FOR MULTIPLE EFFECT EVAPORATORS 76) inventor: Ferris C. Standiford, 2713 S. North Bluff Road, Greenbank, Wash. 9823 22 Filed: Mar. 7, 197 21
United States Patent 19
 United States Patent 19 USOO5853046A 11 Patent Number: 5,853,046 Williams et al. (45) Date of Patent: Dec. 29, 1998 54) HEAT EXCHANGER SEAL APPARATUS 4.914,929 4/1990 Shimazaki. 5,036,931 8/1991 Iritani.
United States Patent 19 USOO5853046A 11 Patent Number: 5,853,046 Williams et al. (45) Date of Patent: Dec. 29, 1998 54) HEAT EXCHANGER SEAL APPARATUS 4.914,929 4/1990 Shimazaki. 5,036,931 8/1991 Iritani.
Cain (45) Date of Patent: May 24, METHOD AND APPARATUS FOR FIBER 4,514,205 4/1985 Darcangelo et al... 65/12
 United States Patent (19) 11 USOO53115A Patent Number: 5,314,515 Cain () Date of Patent: May 24, 1994 54 METHOD AND APPARATUS FOR FIBER 4,514,205 4/1985 Darcangelo et al.... /12 COOLNG 4,583,485 4/986
United States Patent (19) 11 USOO53115A Patent Number: 5,314,515 Cain () Date of Patent: May 24, 1994 54 METHOD AND APPARATUS FOR FIBER 4,514,205 4/1985 Darcangelo et al.... /12 COOLNG 4,583,485 4/986
April 8, 1952 F. W. EDWARDs 2,592,400 HEATER. INVENTOR. Z2-a/aa227A 2.27te2/-23, leadopt ul. "feuwaa Stavvula. a?7215/yat-s.
 April 8, 192 F. W. EDWARDs HEATER Filed June 10, 1946 3. Sheets-Sheet l leadopt ul INVENTOR. Z2-a/aa227A 2.27te2/-23, "feuwaa Stavvula a?721/yat-s. April 8, 192 April 8, 192 Filed June 10, 1946 F. W. EDWARDS
April 8, 192 F. W. EDWARDs HEATER Filed June 10, 1946 3. Sheets-Sheet l leadopt ul INVENTOR. Z2-a/aa227A 2.27te2/-23, "feuwaa Stavvula a?721/yat-s. April 8, 192 April 8, 192 Filed June 10, 1946 F. W. EDWARDS
Nov. 8, 1966 N, GANIARIs 3,283,522
 Nov. 8, 1966 N, GANIARIs FREEZE CONCENTRATION Filed Nov. 4, 1963 2 Sheets-Sheet. 97 96 (e) COMPRESSOR 98. 96 92Y 93 MAN COMPRESSOR 94 29 28 22 23 --- 94. 22 (W) 24 N S. COOLER ETER CONDENSER 9. 8 - Ness
Nov. 8, 1966 N, GANIARIs FREEZE CONCENTRATION Filed Nov. 4, 1963 2 Sheets-Sheet. 97 96 (e) COMPRESSOR 98. 96 92Y 93 MAN COMPRESSOR 94 29 28 22 23 --- 94. 22 (W) 24 N S. COOLER ETER CONDENSER 9. 8 - Ness
Kaminski (45) Date of Patent: Dec. 1, ) Assignee: Owens-Illinois Plastic Products, Inc., 57) ABSTRACT
 United States Patent (19) (11 USOO567316A Patent Number: Kaminski (45) Date of Patent: Dec. 1, 1992 (54) POSITIONING AND INDEXING MOLDED HOLLOW PASTIC ARTICLES 75 Inventor: Ronald S. Kaminski, Bowling
United States Patent (19) (11 USOO567316A Patent Number: Kaminski (45) Date of Patent: Dec. 1, 1992 (54) POSITIONING AND INDEXING MOLDED HOLLOW PASTIC ARTICLES 75 Inventor: Ronald S. Kaminski, Bowling
US 7488,416 B2. Feb. 10, (45) Date of Patent: (10) Patent No.: Chen. people to drink or gargle. The circulating Systems are con
 USOO7488416B2 (12) United States Patent Chen (10) Patent No.: (45) Date of Patent: Feb. 10, 2009 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) (56) BATHING POOLASSEMBLY WITH WATER FULL OF NANO-SCALE
USOO7488416B2 (12) United States Patent Chen (10) Patent No.: (45) Date of Patent: Feb. 10, 2009 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) (56) BATHING POOLASSEMBLY WITH WATER FULL OF NANO-SCALE
(12) United States Patent
 USOO9440513B2 (12) United States Patent Boudard et al. (10) Patent No.: (45) Date of Patent: US 9.440,513 B2 Sep. 13, 2016 (54) ABSORPTION PLATE FOR AN AIR CONDITIONER (75) Inventors: Emmanuel Boudard,
USOO9440513B2 (12) United States Patent Boudard et al. (10) Patent No.: (45) Date of Patent: US 9.440,513 B2 Sep. 13, 2016 (54) ABSORPTION PLATE FOR AN AIR CONDITIONER (75) Inventors: Emmanuel Boudard,
(12) United States Patent (10) Patent No.: US 6,524,394 B2
 USOO6524394B2 (12) United States Patent (10) Patent No.: Okazawa et al. (45) Date of Patent: Feb. 25, 2003 (54) DRY ICE CLEANING METHOD AND DRY 5,025,597 A 6/1991 Tada et al.... 451/39 ICE CLEANING APPARATUS
USOO6524394B2 (12) United States Patent (10) Patent No.: Okazawa et al. (45) Date of Patent: Feb. 25, 2003 (54) DRY ICE CLEANING METHOD AND DRY 5,025,597 A 6/1991 Tada et al.... 451/39 ICE CLEANING APPARATUS
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States US 20130298579A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0298579 A1 Dingle et al. (43) Pub. Date: (54) VAPOR COMPRESSION DEHUMIDIFIER (52) U.S. Cl. USPC... 62/90;
(19) United States US 20130298579A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0298579 A1 Dingle et al. (43) Pub. Date: (54) VAPOR COMPRESSION DEHUMIDIFIER (52) U.S. Cl. USPC... 62/90;
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 20060277782A1 (12) Patent Application Publication (10) Pub. No.: Chen et al. (43) Pub. Date: Dec. 14, 2006 (54) NEGATIVE PRESSURE TYPE DRYING MACHINE THAT UTILIZES THE ENERGY OF THE
(19) United States US 20060277782A1 (12) Patent Application Publication (10) Pub. No.: Chen et al. (43) Pub. Date: Dec. 14, 2006 (54) NEGATIVE PRESSURE TYPE DRYING MACHINE THAT UTILIZES THE ENERGY OF THE
(12) United States Patent
 US007 145105B2 (12) United States Patent Gaullard (10) Patent No.: (45) Date of Patent: Dec. 5, 2006 (54) ELECTRIC KETTLE (75) Inventor: Hervé Gaullard, Courtefontaine (FR) (73) Assignee: SEB SA, Ecully
US007 145105B2 (12) United States Patent Gaullard (10) Patent No.: (45) Date of Patent: Dec. 5, 2006 (54) ELECTRIC KETTLE (75) Inventor: Hervé Gaullard, Courtefontaine (FR) (73) Assignee: SEB SA, Ecully
(12) United States Patent
 US0071 17888B2 (12) United States Patent Niekolaas (10) Patent No.: (45) Date of Patent: Oct. 10, 2006 (54) HYDRAULIC SEPARATOR (75) Inventor: Simon Eduard Niekolaas, Schipluiden (NL) (73) Assignee: Flamco
US0071 17888B2 (12) United States Patent Niekolaas (10) Patent No.: (45) Date of Patent: Oct. 10, 2006 (54) HYDRAULIC SEPARATOR (75) Inventor: Simon Eduard Niekolaas, Schipluiden (NL) (73) Assignee: Flamco
(12) United States Patent (10) Patent No.: US 7,654,310 B2. Li (45) Date of Patent: Feb. 2, 2010
 USOO765431 OB2 (12) United States Patent (10) Patent No.: Li (45) Date of Patent: Feb. 2, 2010 (54) LOOP HEAT PIPE 6,840,304 B1* 1/2005 Kobayashi et al.... 165,111 7,231,961 B2 * 6/2007 Alex et al....
USOO765431 OB2 (12) United States Patent (10) Patent No.: Li (45) Date of Patent: Feb. 2, 2010 (54) LOOP HEAT PIPE 6,840,304 B1* 1/2005 Kobayashi et al.... 165,111 7,231,961 B2 * 6/2007 Alex et al....
5 Separation Processes
 5 Separation Processes 5.1 Distillation 1 Distillation separates chemicals by the difference in how easily they vaporize. The two major types of classical distillation include continuous distillation and
5 Separation Processes 5.1 Distillation 1 Distillation separates chemicals by the difference in how easily they vaporize. The two major types of classical distillation include continuous distillation and
United States Patent (19) Sato
 United States Patent (19) Sato 54 METHOD AND APPARATUS FORVACUUM DRYING COLLODAL SUBSTANCES (75) (73) Inventor: Assignee: Takuya Sato, Suita, Japan Sato Iron Works Co., Ltd., Osaka, Japan (21) Appl. No.:
United States Patent (19) Sato 54 METHOD AND APPARATUS FORVACUUM DRYING COLLODAL SUBSTANCES (75) (73) Inventor: Assignee: Takuya Sato, Suita, Japan Sato Iron Works Co., Ltd., Osaka, Japan (21) Appl. No.:
Sept. 16, ,851,776 J. CZULAK ETAL CHEESE-CURD FUSING MACHINE. Filed March 25, Sheets-Sheet
 Sept. 16, 1958 Filed March 25, 1957 J. CZULAK ETAL CHEESE-CURD FUSING MACHINE 3. Sheets-Sheet Sept. 16, 1958 J. CZULAK ETAL CHEESE-CURD FUSNG MACHINE 3. Sheets-Sheet 2 des o o os o go Sept. 16, 1958 J.
Sept. 16, 1958 Filed March 25, 1957 J. CZULAK ETAL CHEESE-CURD FUSING MACHINE 3. Sheets-Sheet Sept. 16, 1958 J. CZULAK ETAL CHEESE-CURD FUSNG MACHINE 3. Sheets-Sheet 2 des o o os o go Sept. 16, 1958 J.
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 2008.0005926A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0005926 A1 Goggin (43) Pub. Date: Jan. 10, 2008 (54) APPARATUS AND METHOD FOR REDUCING CLOTHES DRYER LINT
US 2008.0005926A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0005926 A1 Goggin (43) Pub. Date: Jan. 10, 2008 (54) APPARATUS AND METHOD FOR REDUCING CLOTHES DRYER LINT
IIIHHHHHHHHHHHHH. United States Patent (19) CSi. 11 Patent Number: 5,318,230 (45) Date of Patent: Jun. 7, Ferguson et al.
 United States Patent (19) Ferguson et al. 54 GARBAGE DISPOSAL ASSEMBLY WITH DECORATIVE SINK FLANGE MASK 75 Inventors: Lloyd G. Ferguson, Marietta, Ga.; Peter J. Taylor, Bishops Wood, United Kingdom 73)
United States Patent (19) Ferguson et al. 54 GARBAGE DISPOSAL ASSEMBLY WITH DECORATIVE SINK FLANGE MASK 75 Inventors: Lloyd G. Ferguson, Marietta, Ga.; Peter J. Taylor, Bishops Wood, United Kingdom 73)
(12) United States Patent (10) Patent No.: US 6,692,130 B1
 USOO6692130B1 (12) United States Patent (10) Patent No.: Snow (45) Date of Patent: Feb. 17, 2004 (54) SOLAR POWERED HEATING AND 5,433,660 A 7/1995 Ohba VENTILATION SYSTEM FOR VEHICLE 5,588.909 A 12/1996
USOO6692130B1 (12) United States Patent (10) Patent No.: Snow (45) Date of Patent: Feb. 17, 2004 (54) SOLAR POWERED HEATING AND 5,433,660 A 7/1995 Ohba VENTILATION SYSTEM FOR VEHICLE 5,588.909 A 12/1996
(12) United States Patent
 (12) United States Patent USOO6898867B1 (10) Patent No. US 6,898,867 B1 VanderPyl (45) Date of Patent May 31, 2005 (54) AIR COMPRESSION VARIABLE HEATING 4,817,387 A * 4/1989 Lashbrook... 60/611 SYSTEM
(12) United States Patent USOO6898867B1 (10) Patent No. US 6,898,867 B1 VanderPyl (45) Date of Patent May 31, 2005 (54) AIR COMPRESSION VARIABLE HEATING 4,817,387 A * 4/1989 Lashbrook... 60/611 SYSTEM
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 20060266O74A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0266074 A1 Groll et al. (43) Pub. Date: (54) HEAT PUMP SYSTEM WITH MULTI-STAGE COMPRESSION (75) Inventors:
(19) United States US 20060266O74A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0266074 A1 Groll et al. (43) Pub. Date: (54) HEAT PUMP SYSTEM WITH MULTI-STAGE COMPRESSION (75) Inventors:
(12) United States Patent (10) Patent No.: US 6,722,866 B1
 USOO6722866B1 (12) United States Patent (10) Patent No.: Dresler (45) Date of Patent: Apr. 20, 2004 (54) PUMP SYSTEM FOR DELIVERING 4,495,855. A 1/1985 Murakami et al.... 92/71 CRYOGENIC LIQUIDS 4,639,197
USOO6722866B1 (12) United States Patent (10) Patent No.: Dresler (45) Date of Patent: Apr. 20, 2004 (54) PUMP SYSTEM FOR DELIVERING 4,495,855. A 1/1985 Murakami et al.... 92/71 CRYOGENIC LIQUIDS 4,639,197
D.C. (21) Appl. No.: 727,081. (22 Filed: Sep. 27, Int. C... G21C 13/10 52 U.S. C /87; 176/38; 52/ /DIG
 United States Patent (19) Wampole (54) 75 73 NUCLEAR REACTOR INSULATION AND PREHEAT SYSTEM Inventor: Assignee: Nevin C. Wampole, Latrobe, Pa. The United States of America as represented by the United States
United States Patent (19) Wampole (54) 75 73 NUCLEAR REACTOR INSULATION AND PREHEAT SYSTEM Inventor: Assignee: Nevin C. Wampole, Latrobe, Pa. The United States of America as represented by the United States
(12) United States Patent
 USOO961 1584B2 (12) United States Patent Choi (10) Patent No.: (45) Date of Patent: Apr. 4, 2017 (54) ELECTRIC IRON WITH ULTRAVIOLET STEAM DISINFECTION FUNCTION (71) Applicant: Lung Wai Choi, Hong Kong
USOO961 1584B2 (12) United States Patent Choi (10) Patent No.: (45) Date of Patent: Apr. 4, 2017 (54) ELECTRIC IRON WITH ULTRAVIOLET STEAM DISINFECTION FUNCTION (71) Applicant: Lung Wai Choi, Hong Kong
(12) United States Patent (10) Patent No.: US 6,629,428 B1
 USOO6629428B1 (12) United States Patent (10) Patent No.: Murry (45) Date of Patent: Oct. 7, 2003 (54) METHOD OF HEATING FOR AN AIRCRAFT 4,503,666 A 3/1985 Christoff... 60/39.07 ELECTRIC ENVIRONMENTAL CONTROL
USOO6629428B1 (12) United States Patent (10) Patent No.: Murry (45) Date of Patent: Oct. 7, 2003 (54) METHOD OF HEATING FOR AN AIRCRAFT 4,503,666 A 3/1985 Christoff... 60/39.07 ELECTRIC ENVIRONMENTAL CONTROL
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 US 20130193219A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0193219 A1 Xia et al. (43) Pub. Date: Aug. 1, 2013 (54) LOW PRESSURE AND HIGH-LOW (52) U.S. Cl. TEMPERATURE
US 20130193219A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0193219 A1 Xia et al. (43) Pub. Date: Aug. 1, 2013 (54) LOW PRESSURE AND HIGH-LOW (52) U.S. Cl. TEMPERATURE
United States Patent (19)
 United States Patent (19) Bergquist (54) SPRAY DRYER (75) Inventor: Dwight H. Bergquist, Omaha, Nebr. 73) Assignee: Henningsen Foods, Inc., White Plains, N.Y. (21) Appl. No.: 247,924 22 Filed: Mar. 26,
United States Patent (19) Bergquist (54) SPRAY DRYER (75) Inventor: Dwight H. Bergquist, Omaha, Nebr. 73) Assignee: Henningsen Foods, Inc., White Plains, N.Y. (21) Appl. No.: 247,924 22 Filed: Mar. 26,
(12) United States Patent
 (12) United States Patent US006 173454B1 (10) Patent No.: US 6,173,454 B1 Alvarez (45) Date of Patent: Jan. 16, 2001 (54) JONNISAFE 5,191,991 3/1993 Jackson... 220/207 5,347,663 9/1994 Yost... 4/253 (76)
(12) United States Patent US006 173454B1 (10) Patent No.: US 6,173,454 B1 Alvarez (45) Date of Patent: Jan. 16, 2001 (54) JONNISAFE 5,191,991 3/1993 Jackson... 220/207 5,347,663 9/1994 Yost... 4/253 (76)
(12) United States Patent (10) Patent No.: US 8,776,539 B2
 US008776539B2 (12) United States Patent (10) Patent No.: US 8,776,539 B2 Verma et al. (45) Date of Patent: Jul. 15, 2014 (54) EJECTOR-TYPE REFRIGERATION CYCLE USPC... 62/115,500,510, 503, 509, 513; AND
US008776539B2 (12) United States Patent (10) Patent No.: US 8,776,539 B2 Verma et al. (45) Date of Patent: Jul. 15, 2014 (54) EJECTOR-TYPE REFRIGERATION CYCLE USPC... 62/115,500,510, 503, 509, 513; AND
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 20160047597A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0047597 A1 Brett et al. (43) Pub. Date: Feb. 18, 2016 (54) METHOD AND APPARATUS INA (30) Foreign Application
(19) United States US 20160047597A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0047597 A1 Brett et al. (43) Pub. Date: Feb. 18, 2016 (54) METHOD AND APPARATUS INA (30) Foreign Application
