WARNINGS AND PRECAUTIONS FOR THE USE OF THE IQ TM Intelligent Driver
|
|
|
- Ambrose Richards
- 6 years ago
- Views:
Transcription
1 Tradeport Drive Jacksonville, FL Revision: A Tel FAX Date: 4/2013 WARNINGS AND PRECAUTIONS FOR THE USE OF THE IQ TM Intelligent Driver ATTENTION OPERATING SURGEON DESCRIPTION Biomet Microfixation manufactures and distributes a variety of instruments to aid in the implantation of internal fixation devices. The Biomet Microfixation IQ Intelligent Driver is a handheld cordless device designed for two functions: drill pilot holes, high-speed insertion of 1.5x4mm titanium fixation screws. The device only operates in the forward direction. The IQ Intelligent Driver is indicated to aid in the implantation of screws (drilling holes and inserting screws). DIRECTIONS FOR USE Maximum value and dependable service may be obtained from your Biomet Microfixation IQ Driver by becoming familiar with the provided maintenance and operation instructions. Please note that these instructions may be different from other manufacturer s drivers. CLEANING AND STERILIZATION OF DRIVER This driver must be cleaned and sterilized after every patient using the following guidelines: Battery must be removed from the handpiece before cleaning and sterilizing Hand wash only Do not exceed 275 degrees F (135 degrees C). Do not submerge in any solution. Do not use ultrasonic cleaners. Do not use surface disinfectants. 1. Ensure that the driver blade/drill has been removed. 2. Remove the battery from the driver and discard. The battery packs are single use and must be removed prior to cleaning and sterilization or damage to the unit may occur. 3. Carefully inspect all devices for flaws or damage prior to sterilization. Devices with flaws or damage should be returned to Biomet Microfixation. 4. Clean the exterior of the driver with a damp towelette to remove any debris. All soiled surfaces should be washed with a neutral (7.0) ph detergent. Use a soft nylon brush to remove any visible debris. Do not submerge driver. 5. Thoroughly rinse the driver with distilled or soft tap water. 6. Sterilization Method*: Pre-Vacuum Steam Autoclave (wrapped): (1) Place driver in the supplied tray. (2) Wrap tray with a Sterilization wrap. (3) Temperature: 270 o (132 o C) (4) Dwell Time: 4 minutes (5) Wrapped Drying Time: 30 minutes minimum (6) Remove from the autoclave immediately after the cycle is complete, and allow the driver to cool for at least 1 hour. STERRAD (Do NOT sterilize any implants AND instrumentation in any STERRAD system) (1) All items including trays must be cleaned, rinsed, and thoroughly dried before loading into the sterilizer. Warning: Failure to ensure that the devices are completely dry before they are processed may result in residual hydrogen peroxide being present on the outer surface of the load. This may cause contact burns when the surface of the load is handled. (2) Carefully inspect all devices for cleanliness and dryness prior to preparing for sterilization. If visible soil is present, the item must be re-cleaned and dried prior to sterilization. If moisture is present, dry the item thoroughly prior to sterilization. (3) Place driver in the supplied tray. (4) Wrap tray in STERRAD compatible wrap. (5) See table below for validated STERRAD systems and the corresponding recommended cycles: Warning: Loads containing moisture may result in either NON-STERILE device or cycle cancellation. Wear chemical resistant gloves when handling items from any load containing moisture. STERRAD System Cycle (US) Cycle (OUS) Cycle Times STERRAD Minutes STERRAD Short 75 Minutes STERRAD 100S - Short 55 Minutes STERRAD NX Standard Standard 28 Minutes STERRAD 100NX Standard Standard 47 Minutes Please refer to the STERRAD Systems User s Guide for general processing instructions, including proper cleaning and drying, and packaging information prior to reprocessing any device in a STERRAD System. Note: After repeated STERRAD Sterilizations, slight discoloration may occur. Health care personnel bear the ultimate responsibility for ensuring that any packaging method or material, including a reusable rigid container system, is suitable for use in sterilization processing and sterility maintenance in a particular health care facility. ing should be conducted in the health care facility to assure that conditions essential to sterilization can be achieved. Since Biomet Microfixation is not familiar with individual hospital handling methods and bioburden, Biomet Microfixation cannot assume responsibility for sterility even though the guideline is followed. Failure to follow these precautionary measures may cause driver failure, or patient injury, and may void warranty. Failure to follow proper infection control guidelines, manufacturing guidelines, or both when disposing of accessory items or cleaning materials may result in hazard to environment. Consult with local authorities for the proper manner of disposal of this device when it is no longer repairable or has otherwise reached the end of its service life. Failure to dispose of this device in the proper manner may result in hazard to the environment. BATTERY REQUIREMENTS This driver uses one battery pack (P/N ) which is solely supplied by Biomet Microfixation. ACCESSORIES The following accessories can be used in conjunction with the IQ device.
2 IQ Driver Battery (P/N ) IQ Drills IQ 1.5mm blade for the insertion of titanium screws OPERATION STERILE BATTERY INSTALLATION AND REMOVAL Insert the battery pack into the driver. Make sure the battery connection on the pack is aligned with the battery connection in the driver. The blue LED lights on the push button will illuminate when the battery pack is properly engaged in the device. To remove the battery, depress the tabs on the sides of the battery pack and pull the battery out. Discard the battery. Do not use excessive force when inserting the IQ battery into the device. If the battery does not insert easily into the device, remove it and re-insert the battery. Failure to properly insert the battery into the device can result in damage to the driver and/or the battery. DRIVER BLADE OR DRILL INSTALLATION AND REMOVAL The IQ Driver is designed to accept only Biomet Microfixation IQ driver blades and drills. Do not attempt to use any other tools in this driver. To install a blade or drill: 1) Push the collet forward and insert the blade/drill into the collet by aligning the wings on the blade/drill with the slots on the driver. 2) While holding the collet in the forward position, apply a slight inward pressure to the blade/drill and rotate the blade/drill clockwise until the etched lines on the blade/drill are aligned with the etched lines on the collet. 3) Release the collet 4) Give a firm pull on the blade/drill to ensure it is properly engaged. To remove a blade or drill: 1) Push the collet forward and hold while rotating the blade/drill counter-clockwise until the wings become aligned with the slots in the driver. 2) Pull the blade/drill straight out. The IQ driver has bit sensing software integrated into the device to decipher whether a drill bit or titanium screw blade is properly inserted into the collet. The device requires 3 seconds to recognize the blade/drill that has been inserted before it will be ready to fire. The driver has one push button switch to activate the device. Always ensure that the driver blade/drill is properly inserted into the collet by giving it a firm pull before use. Failure to properly insert a driver bit may result in injury to the patient, the user, or third party, damage to the driver, and may void the warranty. DRILL MODE The IQ driver will drill pilot holes using drills available from Biomet Microfixation that are intended to be used with the IQ driver. Once a drill bit is properly inserted into the collet, the IQ device is activated and can be fired by pushing and holding the button. When the button is depressed, the drill will rotate in the clockwise rotation at a speed of approximately 10,000 RPM. Utilizing a peck drill technique will help to ensure the device performs as intended. The device is designed to temporarily shut down after it has been running for 5 seconds of constant use to avoid overheating. The IQ Device is active when a drill bit is properly inserted into the collet. Take precautions when handling the device as accidental activation of the button may result in injury to the patient, the user, or third party. If the device shuts down after the 5 second interval while the bit is still engaged in the bone, re-activate the device to facilitate removing the drill from the bone Titanium Screw Mode The IQ driver drives 1.5 X 4mm titanium cross-drive self-drilling screws available from Biomet Microfixation. The device has a two-step activation mechanism to activate such that the device will not fire unless the device is plunged fully. Once a screw blade is properly inserted into the collet follow the instructions below to drive a titanium screw: 1) Load screw onto blade (Note: ensure finger is not located on button to prevent device activation during screw loading, and ensure screw is loaded adequately onto the blade). 2) Contour plate to bone and hold plate securely to the bone with free-hand or plate holding wand. 3) Place screw tip into the center of the plate hole and hold the device perpendicular to the bone for optimum performance 4) Plunge (pre-load) the device fully (tactile feel that the device can t be plunged downward any further). 5) Engage the button to activate (the button is not required to be held down during the full cycle). The internal software in the device is designed to control the motor to prevent excessive torque and ensure the screw seats successfully. 6) Once the screw is fully seated, the blade should be rocked off to disengage from the screw head. If a screw is driven and not seated fully, a hand-held driver and blade can be used to engage the screw and seat it completely. If the device is fired while loading a screw from the screw dial, the screw should be discarded and not implanted. The driver automatically stops when a screw is seated onto a plate. Intraoperative fracture or stripping of screws can occur if additional activations of the device are reapplied. The IQ Device is not recommended for inserting Titanium screws into HTR implant material. WARNINGS The IQ Driver should only be used for the applications listed in the operation section. Use of the device with other systems or lengths of screws can involve incalculable risks for the implant and the driver, thereby potentially endangering the patient, user, or third party. Correct care, handling and maintenance procedures must be maintained to assure proper function. Prior to use, inspect device. If the instrument shows visible signs of damage, the exterior coating shows any sign of degradation or if the device fails to operate properly, discontinue its use immediately and return to the manufacturer. Instruments, which have experienced extensive use or excessive force, may be susceptible to failure. There are no user serviceable components of the IQ device. It must be returned to the manufacturer for repair or service. Care must be taken to avoid over tightening of screws. Intraoperative fracture or stripping of screws can occur if additional activations are applied while seating the screws, or additional torque is provided to the screw using a hand-held driver. While the IQ Driver is expected to aid surgeons in implant insertion, surgeons must also use their judgment to determine if the plunge force of the device exceeds what is acceptable for a certain application. This instrument should only be used for its intended purpose. Correct handling and maintenance of the IQ Driver is important. Substances entering the driver or physical damage to the driver may affect its function. Federal law (USA) restricts this device to sale by or on the order of a physician. Operating Surgeons and all personnel involved with handling these products are responsible for attaining appropriate education and training within the scope of the activities with which they are involved in the handling and use of this product. This equipment contains electronic and ferrous components whose operation can be affected by intense electromagnetic fields. Do not operate the IQ Driver in an MRI environment or in the vicinity of high-frequency surgical diathermy equipment, defibrillators, or shortwave therapy equipment. Electromagnetic interference could disrupt the operation of the IQ Driver. This equipment is specified for use only in shielded location.
3 This equipment generates, uses, and can radiate radio frequency energy. If not installed and used in accordance with the instructions in this manual, electromagnetic interference may result. The equipment has been tested and found to comply with the limits set forth in IEC for Medical Products. These limits provide reasonable protection against electromagnetic interference when operated in the intended use environments described in this manual. TROUBLESHOOTING Driver will not activate Blade/drill falls out. Driver fires inadvertently Driver fires intermittently or incorrectly Driver bogs down during drilling Driver will not activate. Ensure the device is given 3 seconds to recognize the blade/drill after inserted into collet. Battery may be loose. Make sure battery is pushed in all the way and the button is lit. Battery drained. Always use a fresh battery. Driver blade/drill not properly inserted. Pull on bit to be certain it is secure or remove bit and re-engage bit into collet. Driver blade/drill inserted prior to the battery. Remove the blade/drill and ensure the device is given 3 seconds to reset and then re-insert the blade/drill and given another 3 seconds to recognize the blade/drill after inserted into the collet. Blade/drill not properly inserted. Pull on bit to be certain it is secure. Ensure finger is not placed on button during drill mode, or when inserting a blade/drill into the collet. If device fires without finger on button, discontinue use and contact the manufacturer. Remove the battery and remove the blade/drill engaged in collet. Reinsert the battery and blade/drill. If problem persists, discontinue use and contact Biomet Microfixation. Remove the battery and remove the blade/drill engaged in collet. Reinsert the battery and blade/drill. If problem persists, discontinue use and contact Biomet Microfixation. Replace battery and/or replace drill bit. If problem persists, discontinue use and contact manufacturer. Utilize a peck drilling technique. If problem persists, discontinue use and contact manufacturer. Titanium screw doesn t seat fully Titanium screw strips out the bone Blade strips titanium screw head Ensure the plate is contoured to the bone, the screw is centered in the plate hole and the screw is axial to the blade during insertion for optimal performance. Ensure a new battery was used at the beginning of the case. For less dense bone, manually insert screws. Ensure the screw is loaded onto the blade with proper alignment and retention. Ensure the screw is axial to the blade during insertion. MAINTENANCE The driver requires no regular maintenance. TECHNICAL SPECIFICATIONS Product Number: Classification: Internally powered, Type B applied part Rated battery Voltage: Input ratings: A Mode of operation: Intermittent 25% On time Suitability for use with flammable anesthetics: Equipment not suitable for use in presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide. Shipping and storage conditions: F (5 50 C): 20 95% Relative Humidity (RH) Guidance and manufacturer's declaration- electromagnetic emissions Emissions RF emissions CISPR 11 RF emissions CISPR 11 Harmonic emissions IEC Voltage fluctuations/ flicker emissions IEC Group 1 Class B Not applicable Not applicable Electromagnetic environment- guidance The IQ Intelligent driver uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. The IQ Intelligent driver is suitable for use in all establishments, including domestic establishments and those directly connected to the public lowvoltage power supply network that supplies buildings used for domestic purposes.
4 3 Table 1: Guidance and Manufacturer s Declaration-electromagnetic Emissions The manufacturer recommends usage of the IQ driver within domestic establishments specific to a hospital operating room. Guidance and manufacturer's declaration- electromagnetic immunity IEC test level Electromagnetic environmentguidance Electrosta tic ± 6 kv discharge ± 6 kv contact contact (ESD) IEC ± 8 kv air ± 8 kv air Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Table 2: Guidance and Manufacturer s Declaration- Electromagnetic Guidance and manufacturer's declaration- electromagnetic immunity IEC test level Electromagnetic environment- guidance Recommended separation distance d= 1.17 P 80 MHz to 800 MHz d=2.33 P 800 MHz to 1.5 GHz Radiated RF IEC V/m 80 MHz to 2.5 GHz 3 V/m 80 MHz to 2.5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in metres (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic survey, a should be less than the compliance level in each frequency range. b Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1: At 80 MHZ and 800 MHz, the higher frequency range applies. NOTE 2: These guidelines might not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmiters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the IQ Intelligent driver is used exceeds the applicable RF compliance level abone, the IQ Intelligent driver should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the IQ Intelligent driver. b Over the frequency range 150 khz to 80 MHz, field strengths should be less than [V 1] V/m. Table 3: Guidance and Manufacturer s Declaration- Electromagnetic PRODUCT WARRANTY The Biomet Microfixation IQ Intelligent Driver is warranted to be free from defects in material and workmanship for a period of one (1) year from date of purchase. This warranty applies only if the product has been maintained per these instructions. The warranty will not apply if the product has been subject to misuse or abuse. Sole liability under this warranty is limited to the repair or replacement, at our option, of the defective product. No other warranties are expressed or implied. Any defective product must be returned to the address below with proof of purchase (copy of dealer invoice) and transportation prepaid. IQ is a trademark of Biomet, patent pending. Biomet and all other trademarks herein are the property of Biomet, Inc. or its subsidiaries. Authorized Representative in EC: European Sales Headquarters: Biomet UK, Ltd. Biomet Microfixation Europe Waterton Industrial Estates, Toermalijnring LC Bridgend, South Wales 3316 LC Dordrechtv CF31 3XA, U.K. Netherlands Phone: (+44) Phone: (+31)
5 Fax: (+44) Fax: (+31)
Enhance DX. Mattress Replacement System. User Manual
 Enhance DX Mattress Replacement System User Manual Prius Healthcare USA 580 Corporate Center 4025 Tampa Road, #1117 Oldsmar, FL 34677, USA TEL: (813)854-5464 FAX: (813)854-5442 1111 Content 1. THE PURPOSE
Enhance DX Mattress Replacement System User Manual Prius Healthcare USA 580 Corporate Center 4025 Tampa Road, #1117 Oldsmar, FL 34677, USA TEL: (813)854-5464 FAX: (813)854-5442 1111 Content 1. THE PURPOSE
Instructions for use / Alarm unit, Fiber optic cable & Sensor patch ENGLISH
 Instructions for use / Alarm unit, Fiber optic cable & Sensor patch ENGLISH Manufacturer: Redsense Medical AB Gyllenhammars väg 26 302 92 HALMSTAD SWEDEN www.redsensemedical.com These instructions are
Instructions for use / Alarm unit, Fiber optic cable & Sensor patch ENGLISH Manufacturer: Redsense Medical AB Gyllenhammars väg 26 302 92 HALMSTAD SWEDEN www.redsensemedical.com These instructions are
Transcend Heated Humidifier User Manual
 Transcend Heated Humidifier User Manual Page 1 Transcend Heated Humidifier TM User Manual Notices Revised Notice Trademark Transcend Heated Humidifier User Manual 103404 Rev H Published February 2016
Transcend Heated Humidifier User Manual Page 1 Transcend Heated Humidifier TM User Manual Notices Revised Notice Trademark Transcend Heated Humidifier User Manual 103404 Rev H Published February 2016
Veterinary Patient Warming System Controller User Manual
 Veterinary Patient Warming System Controller User Manual Contents Introduction... 3 Indications for use... 3 Contraindications... 3 Warnings... 3 Precautions... 4 Proper Use and Maintenance... 5 Initial
Veterinary Patient Warming System Controller User Manual Contents Introduction... 3 Indications for use... 3 Contraindications... 3 Warnings... 3 Precautions... 4 Proper Use and Maintenance... 5 Initial
CLETEF-786. Infrared Thermometer Instruction Manual
 CLETEF-786 0482 Infrared Thermometer Instruction Manual Introduction Thank you for choosing the Clever Choice Duo TM Ear & Forehead Thermometer, by Simple Diagnostics. The infrared thermometer CLETEF-786
CLETEF-786 0482 Infrared Thermometer Instruction Manual Introduction Thank you for choosing the Clever Choice Duo TM Ear & Forehead Thermometer, by Simple Diagnostics. The infrared thermometer CLETEF-786
ASPIRE Laboratory Aspirator
 ASPIRE Laboratory Aspirator USER MANUAL Rev 2/14/18 Accuris Instruments / Benchmark Scientific Ph: (908) 769-5555 E-mail: info@accuris-usa.com (C) Benchmark Scientific, 2018 THE ACCURIS ASPIRE LABORATORY
ASPIRE Laboratory Aspirator USER MANUAL Rev 2/14/18 Accuris Instruments / Benchmark Scientific Ph: (908) 769-5555 E-mail: info@accuris-usa.com (C) Benchmark Scientific, 2018 THE ACCURIS ASPIRE LABORATORY
MaxiCompressor. Limited Warranty. High Performance 50 PSI Compressor 501-S
 Limited Warranty Global Medical Holdings (GMH) warrants the MaxiCompressor for 1 year from the date of purchase due to faulty parts or workmanship. This warranty is limited to the original purchaser of
Limited Warranty Global Medical Holdings (GMH) warrants the MaxiCompressor for 1 year from the date of purchase due to faulty parts or workmanship. This warranty is limited to the original purchaser of
Made in USA. Sunoptic Technologies 6018 Bowdendale Avenue Jacksonville, FL 32216, USA (904)
 Fiber Optic Cables Instructions for Use and Processing Instructions DFU-950-0031-00 Fiber Optic Cables Made in USA Sunoptic Technologies 6018 Bowdendale Avenue Jacksonville, FL 32216, USA (904) 737 7611
Fiber Optic Cables Instructions for Use and Processing Instructions DFU-950-0031-00 Fiber Optic Cables Made in USA Sunoptic Technologies 6018 Bowdendale Avenue Jacksonville, FL 32216, USA (904) 737 7611
Table of Contents. English
 OM-E0799E 000 English Thank you for purchasing VIVA ace Motor Kit. Please read this Operation Manual and the VIVA ace Basic Set Operation Manual carefully before use for operating instructions and care
OM-E0799E 000 English Thank you for purchasing VIVA ace Motor Kit. Please read this Operation Manual and the VIVA ace Basic Set Operation Manual carefully before use for operating instructions and care
Veterinary Patient Warming System Service Manual. For information on operating the Hot Dog Patient Warming System, refer to the Instructions for Use
 Veterinary Patient Warming System Service Manual For information on operating the Hot Dog Patient Warming System, refer to the Instructions for Use Hot Dog TM Patient Warming System Service Manual Page
Veterinary Patient Warming System Service Manual For information on operating the Hot Dog Patient Warming System, refer to the Instructions for Use Hot Dog TM Patient Warming System Service Manual Page
EkoSonic Control Unit Instructions for Use
 EkoSonic Control Unit Instructions for Use Caution: Federal (U.S.) law restricts this device to use by or on the order of a physician. 6084-001 REV K Intended Use The EKOS EkoSonic Control Unit is intended
EkoSonic Control Unit Instructions for Use Caution: Federal (U.S.) law restricts this device to use by or on the order of a physician. 6084-001 REV K Intended Use The EKOS EkoSonic Control Unit is intended
ULTRASONIC AIR SALINIZER. Owner s Manual. Saltair Home Salt Therapy
 Saltair Home Salt Therapy ULTRASONIC AIR SALINIZER Owner s Manual Declaration of Conformity: This device was manufactured and controlled by ISO 9001 Certified Management System and conform to all aspects
Saltair Home Salt Therapy ULTRASONIC AIR SALINIZER Owner s Manual Declaration of Conformity: This device was manufactured and controlled by ISO 9001 Certified Management System and conform to all aspects
COOLFLOW IRRIGATION PUMP USER MANUAL
 COOLFLOW IRRIGATION PUMP USER MANUAL Manual P/N: M-5276-323A 2001 Biosense Webster, Inc. All rights reserved. Biosense Webster, Inc. 3333 Diamond Canyon Road Diamond Bar, CA 91765, USA Tel: +1-909-839-8500
COOLFLOW IRRIGATION PUMP USER MANUAL Manual P/N: M-5276-323A 2001 Biosense Webster, Inc. All rights reserved. Biosense Webster, Inc. 3333 Diamond Canyon Road Diamond Bar, CA 91765, USA Tel: +1-909-839-8500
Operator s Guide. Autoclavable Internal Handles with Integrated Paddles Rev. M
 Operator s Guide Autoclavable Internal Handles with Integrated Paddles 9650-0550 Rev. M The issue date for the Autoclavable Internal Handles with Integrated Paddles Operator s Guide (REF 9650-0550 Rev.
Operator s Guide Autoclavable Internal Handles with Integrated Paddles 9650-0550 Rev. M The issue date for the Autoclavable Internal Handles with Integrated Paddles Operator s Guide (REF 9650-0550 Rev.
PEAK Platelet Rich Plasma System
 PEAK Platelet Rich Plasma System 6969-01 Rev. AA 2014-4-25 Disposable Part No. 278001 Page 1 PEAK Platelet Rich Plasma System Instructions for Use (U.S.) System Description The PEAK Platelet Rich Plasma
PEAK Platelet Rich Plasma System 6969-01 Rev. AA 2014-4-25 Disposable Part No. 278001 Page 1 PEAK Platelet Rich Plasma System Instructions for Use (U.S.) System Description The PEAK Platelet Rich Plasma
5. Instrument and accessories discharging must be done following current law regulations in every country of use.
 VEGA it s a device working 230V ~ / 50 Hz network electricity, to be used for the nasal aspiration, oral aspiration, tracheal aspiration of the body liquids (mucus, catarrh or blood) in the adult or in
VEGA it s a device working 230V ~ / 50 Hz network electricity, to be used for the nasal aspiration, oral aspiration, tracheal aspiration of the body liquids (mucus, catarrh or blood) in the adult or in
TM Machine WetAlert TM Wireless Wetness Detector Home User s Guide
 2008K@home TM Machine WetAlert TM Wireless Wetness Detector Home User s Guide 2008K@home WetAlert Wireless Wetness Detector Home User s Guide Copyright 2012-2014, 2016 Fresenius USA, Inc. All Rights Reserved
2008K@home TM Machine WetAlert TM Wireless Wetness Detector Home User s Guide 2008K@home WetAlert Wireless Wetness Detector Home User s Guide Copyright 2012-2014, 2016 Fresenius USA, Inc. All Rights Reserved
HydraTherm TM OPERATION MANUAL
 HydraTherm TM OPERATION MANUAL TABLE OF CONTENTS Warranty... 1 Forward...2 Symbols Glossary...2 Precautionary Instructions...3 Detailed Device Description...4 Recommended Pack Setup...6 Installation...7
HydraTherm TM OPERATION MANUAL TABLE OF CONTENTS Warranty... 1 Forward...2 Symbols Glossary...2 Precautionary Instructions...3 Detailed Device Description...4 Recommended Pack Setup...6 Installation...7
OPERATORS GUIDE Rocket R54569 PSU
 OPERATORS GUIDE Rocket R54569 PSU Portable low vacuum/low flow Suction Pump 1. GENERAL ASSEMBLY 1. Carry Handle 2. On/Off Button 3. Control/Decision interface 4. Back Lit LCD Screen 5. Rotating lock for
OPERATORS GUIDE Rocket R54569 PSU Portable low vacuum/low flow Suction Pump 1. GENERAL ASSEMBLY 1. Carry Handle 2. On/Off Button 3. Control/Decision interface 4. Back Lit LCD Screen 5. Rotating lock for
RESmart CPAP System. User Manual
 RESmart CPAP System User Manual 0123 Table of Contents Symbols... 1 Intended Use... 2 Specifications... 3 Warning & Cautions... 4 Unpacking the System... 5 System Features... 6 First Time Setup... 7 Installing
RESmart CPAP System User Manual 0123 Table of Contents Symbols... 1 Intended Use... 2 Specifications... 3 Warning & Cautions... 4 Unpacking the System... 5 System Features... 6 First Time Setup... 7 Installing
Owner s/service Manual. LED Light Source for A-Series TM. Microscopes M A801-LED
 Owner s/service Manual LED Light Source for A-Series TM Microscopes M A801-LED 110-013-081 REV C ECO 202810 Date Effective: June 2017 When contacting Global Surgical Corporation for either Customer Service
Owner s/service Manual LED Light Source for A-Series TM Microscopes M A801-LED 110-013-081 REV C ECO 202810 Date Effective: June 2017 When contacting Global Surgical Corporation for either Customer Service
Visao HIGH-SPEED OTOLOGIC DRILL CATALOG
 Visao HIGH-SPEED OTOLOGIC DRILL CATALOG Visao High-Speed Otologic Drill with the Integrated Power Console (IPC ) System High speed and torque 80,000 rpm max speed provides rapid, yet precise bone removal
Visao HIGH-SPEED OTOLOGIC DRILL CATALOG Visao High-Speed Otologic Drill with the Integrated Power Console (IPC ) System High speed and torque 80,000 rpm max speed provides rapid, yet precise bone removal
CONTENTS IMPORTANT SAFEGUARDS INTRODUCTION PRODUCT DESCRIPTION INSTALLATION OPERATIONS CLEANING...
 CONTENTS IMPORTANT SAFEGUARDS... 2. INTRODUCTION... 3 2. PRODUCT DESCRIPTION... 4 3. INSTALLATION... 5 4. OPERATIONS... 7 5. CLEANING... 8 6. MATTRESS STORAGE... 9 7. MAINTENANCE... 9 8. TECHNICAL DESCRIPTION...
CONTENTS IMPORTANT SAFEGUARDS... 2. INTRODUCTION... 3 2. PRODUCT DESCRIPTION... 4 3. INSTALLATION... 5 4. OPERATIONS... 7 5. CLEANING... 8 6. MATTRESS STORAGE... 9 7. MAINTENANCE... 9 8. TECHNICAL DESCRIPTION...
Cleaning & Sterilization
 Handpieces & Attachments Instruments Cleaning & Sterilization System 7 System 6 System 5 CD4 CD3 SABO 2 SABO RemB CORE TPS Processing Instructions User/Patient Safety all contribute to the efficacy of
Handpieces & Attachments Instruments Cleaning & Sterilization System 7 System 6 System 5 CD4 CD3 SABO 2 SABO RemB CORE TPS Processing Instructions User/Patient Safety all contribute to the efficacy of
Reusable Laryngoscope Blades and Handles
 Manufacturer: Starkling Medizintechnik Method: Re-processing instructions Symbol: N.A. Device(s): 1 Green System Reusable Laryngoscope Blade with integrated Light Guide, Solid Stainless Steel Construction
Manufacturer: Starkling Medizintechnik Method: Re-processing instructions Symbol: N.A. Device(s): 1 Green System Reusable Laryngoscope Blade with integrated Light Guide, Solid Stainless Steel Construction
CARESTREAM DRX-1 System Detector CARESTREAM DRX-1C System Detector CARESTREAM DRX 2530C Detector Model DRX User Guide
 CARESTREAM DRX-1 System Detector CARESTREAM DRX-1C System Detector CARESTREAM DRX 2530C Detector Model DRX 2530-01 User Guide Publication No.: AA5348 2012-12-20 1 2 AA5348 2012-12-20 CARESTREAM DRX-1 System
CARESTREAM DRX-1 System Detector CARESTREAM DRX-1C System Detector CARESTREAM DRX 2530C Detector Model DRX 2530-01 User Guide Publication No.: AA5348 2012-12-20 1 2 AA5348 2012-12-20 CARESTREAM DRX-1 System
CLEANING AND HANDLING OF WRIGHT INSTRUMENTS
 CLEANING AND HANDLING OF WRIGHT INSTRUMENTS 150824-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
CLEANING AND HANDLING OF WRIGHT INSTRUMENTS 150824-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
System One Heated Humidifier USER MANUAL
 System One Heated Humidifier USER MANUAL 2016 Koninklijke Philips N.V. All rights reserved. Table of Contents Intended Use... 2 Warnings... 2 Cautions... 2 Contraindications... 3 Symbol Key... 3 System
System One Heated Humidifier USER MANUAL 2016 Koninklijke Philips N.V. All rights reserved. Table of Contents Intended Use... 2 Warnings... 2 Cautions... 2 Contraindications... 3 Symbol Key... 3 System
Integra Salto Talaris and Integra XT Non-Sterile Device Reprocessing. Instructions for Use. 4. Manual Cleaning Instructions
 Integra Salto Talaris and Integra XT Non-Sterile Device Reprocessing Instructions for Use. Description and Intended Use Integra LifeSciences Corporation reusable surgical device sets consist of various
Integra Salto Talaris and Integra XT Non-Sterile Device Reprocessing Instructions for Use. Description and Intended Use Integra LifeSciences Corporation reusable surgical device sets consist of various
IMPORTANT INFORMATION ON THE DEPUY SYNTHES
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE DEPUY SYNTHES MatrixNEURO RECONSTRUCTION MESH, STERILE MatrixNEURO PREFORMED MESH, STERILE GP2949-B-CAN DESCRIPTION The DePuy Synthes
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE DEPUY SYNTHES MatrixNEURO RECONSTRUCTION MESH, STERILE MatrixNEURO PREFORMED MESH, STERILE GP2949-B-CAN DESCRIPTION The DePuy Synthes
X99 Series. Dental Handpiece. Instruction For Use
 X99 Series Dental Handpiece Instruction For Use 0413 Table of Contents Symbols... 2 Introduction... 3-4 Before Use... 5 Product description... 6 Technical Specifications... 7 Operation... 8-11 Hygienic
X99 Series Dental Handpiece Instruction For Use 0413 Table of Contents Symbols... 2 Introduction... 3-4 Before Use... 5 Product description... 6 Technical Specifications... 7 Operation... 8-11 Hygienic
REMstar SE USER MANUAL
 REMstar SE USER MANUAL 2012 Koninklijke Philips Electronics N.V. All rights reserved. Table of Contents Intended Use... 2 Important... 2 Warnings... 2 Cautions... 3 Contraindications... 3 Symbol Key...
REMstar SE USER MANUAL 2012 Koninklijke Philips Electronics N.V. All rights reserved. Table of Contents Intended Use... 2 Important... 2 Warnings... 2 Cautions... 3 Contraindications... 3 Symbol Key...
OPERATION MANUAL. Multi Function Ultrasonic Scaler. Varios 970. Please read this Operation Manual carefully before use, and file for future reference.
 Multi Function Ultrasonic Scaler Varios 970 OPERATION MANUAL Please read this Operation Manual carefully before use, and file for future reference. OM-E0464E 001 Classifications of equipment Type of protection
Multi Function Ultrasonic Scaler Varios 970 OPERATION MANUAL Please read this Operation Manual carefully before use, and file for future reference. OM-E0464E 001 Classifications of equipment Type of protection
MODEL 7000 SUCTION UNIT
 MODEL 7000 SUCTION UNIT OPERATOR S MANUAL Caution Federal law restricts this device to sale by or on order of a physician, or any other practitioner licensed by the law of the State in which he practices
MODEL 7000 SUCTION UNIT OPERATOR S MANUAL Caution Federal law restricts this device to sale by or on order of a physician, or any other practitioner licensed by the law of the State in which he practices
Operator s Manual. Video Laryngoscope. ( Instructions for use ) Doc no: Revision 1.1 8th January 2013
 Video Laryngoscope Operator s Manual ( Instructions for use ) Doc no: 100-160-000 Revision 1.1 8th January 2013 Aircraft Medical Ltd 2012 www.aircraftmedical.com Contents 1 Introduction 2 1.1 Description
Video Laryngoscope Operator s Manual ( Instructions for use ) Doc no: 100-160-000 Revision 1.1 8th January 2013 Aircraft Medical Ltd 2012 www.aircraftmedical.com Contents 1 Introduction 2 1.1 Description
Operator s Manual. Model G32-S Model G32-E Disinfection Soak Stations
 Model G32-S Model G32-E Disinfection Soak Stations Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741 Fax: 1-877-329-2482 Website: WWW.CIVCO.COM Copyright
Model G32-S Model G32-E Disinfection Soak Stations Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741 Fax: 1-877-329-2482 Website: WWW.CIVCO.COM Copyright
CARESTREAM DRX-1 System
 Carestream Carestream DRX1 System CARESTREAM DRX-1 System Safety and Regulatory Information with Hardware User s Guide H224_0028HC Version 3.0 PN 7H8166 6 April 2009 Table of Contents 1 Safety and Regulatory
Carestream Carestream DRX1 System CARESTREAM DRX-1 System Safety and Regulatory Information with Hardware User s Guide H224_0028HC Version 3.0 PN 7H8166 6 April 2009 Table of Contents 1 Safety and Regulatory
Operating / User Manual
 UniARM Surgical Support System Operating / User Manual Version 1.1 MITAKA KOHKI Co., Ltd. 1. Introduction Introduction Thank you for purchasing the UniARM for use in surgical procedures. To use the device
UniARM Surgical Support System Operating / User Manual Version 1.1 MITAKA KOHKI Co., Ltd. 1. Introduction Introduction Thank you for purchasing the UniARM for use in surgical procedures. To use the device
Regulatory Information and Specifications
 Regulatory Information and Specifications Introduction This document contains the required regulatory information and specifications for A-dec products. Products Requiring Agency Information Specific regulatory
Regulatory Information and Specifications Introduction This document contains the required regulatory information and specifications for A-dec products. Products Requiring Agency Information Specific regulatory
MyKi Care Thermometer (MCT 1.0) USER MANUAL
 Precautions for use Any form of modification to this device is forbidden; Don t use the device together with MRI or CT equipment; Explosion danger: don t use the device in flammable anesthetic gas; Don
Precautions for use Any form of modification to this device is forbidden; Don t use the device together with MRI or CT equipment; Explosion danger: don t use the device in flammable anesthetic gas; Don
Directions for use MACH LED 300DF
 Directions for use MACH LED 300DF Design with central reflector available against surcharge Dr. Mach GmbH u. Co. KG, Flossmannstrasse 28, D-85560 Ebersberg Tel.: +49 (0)8092 2093 0, Fax +49 (0)8092 2093
Directions for use MACH LED 300DF Design with central reflector available against surcharge Dr. Mach GmbH u. Co. KG, Flossmannstrasse 28, D-85560 Ebersberg Tel.: +49 (0)8092 2093 0, Fax +49 (0)8092 2093
ComPact Air Drive II USER S MANUAL
 ComPact Air Drive II USER S MANUAL R Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF TABLE OF CONTENTS Page ComPact Air Drive II Specifications... 2 Attachments...
ComPact Air Drive II USER S MANUAL R Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF TABLE OF CONTENTS Page ComPact Air Drive II Specifications... 2 Attachments...
Operator s Manual. Model G17-EU (International) Disinfection Soak Station for Hysteroscopes, Cystoscopes and ENT scopes
 Model G17-EU (International) Disinfection Soak Station for Hysteroscopes, Cystoscopes and ENT scopes Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741
Model G17-EU (International) Disinfection Soak Station for Hysteroscopes, Cystoscopes and ENT scopes Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741
CONTENTS CONTROLS 3-STAGE AIR PURIFICATION. X This product is suitable for 120V only. User Manual. Odor sensor. ➌ PlasmaWave
 User Manual CONTENTS 3-Stage Air Purification Controls 3 4 Where to use Installing the s 5 6 Set Up WINIX AIR CLEANER Safety Instructions Safety and Cautions 8 Operation Initial Operation Modes of Operation
User Manual CONTENTS 3-Stage Air Purification Controls 3 4 Where to use Installing the s 5 6 Set Up WINIX AIR CLEANER Safety Instructions Safety and Cautions 8 Operation Initial Operation Modes of Operation
OPERATING INSTRUCTIONS AND MAINTENANCE MANUAL
 OPERATING INSTRUCTIONS AND MAINTENANCE MANUAL S-SCORT II Model 15006 SSCOR, INC. 11064 Randall Street Sun Valley, CA 91352 USA Telephone +1-818-504-4054 800-434-5211 Fax +1-818-504-6032 www.sscor.com Email:
OPERATING INSTRUCTIONS AND MAINTENANCE MANUAL S-SCORT II Model 15006 SSCOR, INC. 11064 Randall Street Sun Valley, CA 91352 USA Telephone +1-818-504-4054 800-434-5211 Fax +1-818-504-6032 www.sscor.com Email:
User manual. DreamStation Heated Humidifier
 ! User manual DreamStation Heated Humidifier! Table of Contents Intended Use... 1 Warnings... 1 Cautions... 1 Contraindications... 2 Symbol Key... 2 System Overview... 2 System Features and Contents...
! User manual DreamStation Heated Humidifier! Table of Contents Intended Use... 1 Warnings... 1 Cautions... 1 Contraindications... 2 Symbol Key... 2 System Overview... 2 System Features and Contents...
Instruction Manual and Warranty Information
 CLEAN MIST TOP-FILL SENSATOUCH HUMIDIFIER WITH REMOTE CONTROL & AROMA TRAY TABLETOP & FLOOR STANDING Instruction Manual and Warranty Information IM0057A READ AND SAVE THESE INSTRUCTIONS Contents Safety
CLEAN MIST TOP-FILL SENSATOUCH HUMIDIFIER WITH REMOTE CONTROL & AROMA TRAY TABLETOP & FLOOR STANDING Instruction Manual and Warranty Information IM0057A READ AND SAVE THESE INSTRUCTIONS Contents Safety
STERITITE INSTRUCTIONS FOR USE
 STERITITE INSTRUCTIONS FOR USE If a rigid container system is used, the manufacturers instructions regarding set preparation and assembly should be followed. -ANSI/AAMI ST79: 2010 & A1:2010 Notes from
STERITITE INSTRUCTIONS FOR USE If a rigid container system is used, the manufacturers instructions regarding set preparation and assembly should be followed. -ANSI/AAMI ST79: 2010 & A1:2010 Notes from
Titan Series Blanket Warmer
 Owner s Manual Titan Series Blanket Warmer EC250 EC350 EC750 EC250 EC350 EC750 Enthermics Medical Systems An ISO 13485:2003 certified company W164 N9221 Water St Menomonee Falls WI 53051 Tel 262-251-8356
Owner s Manual Titan Series Blanket Warmer EC250 EC350 EC750 EC250 EC350 EC750 Enthermics Medical Systems An ISO 13485:2003 certified company W164 N9221 Water St Menomonee Falls WI 53051 Tel 262-251-8356
Cordless Electric Broom
 5328 Series Cordless Electric Broom December 2013 Version 2.05 General Warnings IMPORTANT SAFETY INSTRUCTIONS! When using an electrical appliance, basic precautions should always be followed, including
5328 Series Cordless Electric Broom December 2013 Version 2.05 General Warnings IMPORTANT SAFETY INSTRUCTIONS! When using an electrical appliance, basic precautions should always be followed, including
I.F.U Re/Processing Reusable Medical Devices
 I.F.U Re/Processing Reusable Medical Devices Distributed by: MANUFACTURER: GA 26-09-001 -EN /20130719 Contents 1 Overview of preparation methods... 3 2 Safety and responsibility... 4 3 Explanation of symbols...
I.F.U Re/Processing Reusable Medical Devices Distributed by: MANUFACTURER: GA 26-09-001 -EN /20130719 Contents 1 Overview of preparation methods... 3 2 Safety and responsibility... 4 3 Explanation of symbols...
23-IN Electric Logset
 23-IN Electric Logset ASSEMBLY, CARE & USE INSTRUCTIONS MODEL # ELCG240-INF Questions, problems, missing parts? Before returning to your retailer, call our customer service department at 1-855-571-1044
23-IN Electric Logset ASSEMBLY, CARE & USE INSTRUCTIONS MODEL # ELCG240-INF Questions, problems, missing parts? Before returning to your retailer, call our customer service department at 1-855-571-1044
INSTRUCTIONS FOR CLEANING AND STERILIZATION OF ALL B.BRAUN AESCULAP HAND HELD SURGICAL INSTRUMENTS AND POWER PRODUCTS (UNITED KINGDOM REQUIREMENTS)
 INSTRUCTIONS FOR CLEANING AND STERILIZATION OF ALL B.BRAUN AESCULAP HAND HELD SURGICAL INSTRUMENTS AND POWER PRODUCTS (UNITED KINGDOM REQUIREMENTS) 1 INTRODUCTION This paper is intended to give general
INSTRUCTIONS FOR CLEANING AND STERILIZATION OF ALL B.BRAUN AESCULAP HAND HELD SURGICAL INSTRUMENTS AND POWER PRODUCTS (UNITED KINGDOM REQUIREMENTS) 1 INTRODUCTION This paper is intended to give general
Rigid Reusable & Single use Sigmoidoscopes, Anoscopes, and Accessories ENGLISH 1. Perform reprocessing immediately after each use.
 Rigid Reusable & Single use Sigmoidoscopes, Anoscopes, and Accessories ENGLISH 1 ENGLISH Intended Use The Welch Allyn Rigid Reusable & Single Use Sigmoidoscopes and Anoscopes are used to illuminate and
Rigid Reusable & Single use Sigmoidoscopes, Anoscopes, and Accessories ENGLISH 1 ENGLISH Intended Use The Welch Allyn Rigid Reusable & Single Use Sigmoidoscopes and Anoscopes are used to illuminate and
Concept S-2. Indoor Infrared Stove Heater. RedCore Stove Heater
 Concept S-2 Indoor Infrared Stove Heater RedCore Stove Heater 2011-04-20 CUSTOMER SUPPORT DO NOT RETURN THIS PRODUCT TO THE STORE WHERE YOU BOUGHT IT FOR IMMEDIATE CUSTOMER SERVICE, PLEASE CALL: 888-4BGT
Concept S-2 Indoor Infrared Stove Heater RedCore Stove Heater 2011-04-20 CUSTOMER SUPPORT DO NOT RETURN THIS PRODUCT TO THE STORE WHERE YOU BOUGHT IT FOR IMMEDIATE CUSTOMER SERVICE, PLEASE CALL: 888-4BGT
idrive Ultra Powered Stapling System
 idrive Ultra Powered Stapling System Product Information Kit Stapling Made Smarter Table of Contents Product Introduction 2 System Introduction 3 Features and Benefits 4 System Components 5 Tri-Staple
idrive Ultra Powered Stapling System Product Information Kit Stapling Made Smarter Table of Contents Product Introduction 2 System Introduction 3 Features and Benefits 4 System Components 5 Tri-Staple
CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization
 CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization 1. WARNINGS AND PRECAUTIONS 1.1. Caution should be exercised when handling instruments with sharp points, or cutting/drilling
CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization 1. WARNINGS AND PRECAUTIONS 1.1. Caution should be exercised when handling instruments with sharp points, or cutting/drilling
Whisper. Ultrasonic Leak Detector
 O P E R A T I N G M A N U A L Whisper Ultrasonic Leak Detector Declaration Of Conformity This is to certify that this equipment, designed and manufactured by INFICON Inc., Two Technology Place, East Syracuse,
O P E R A T I N G M A N U A L Whisper Ultrasonic Leak Detector Declaration Of Conformity This is to certify that this equipment, designed and manufactured by INFICON Inc., Two Technology Place, East Syracuse,
CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization
 CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization 1. WARNINGS AND PRECAUTIONS 1.1. Caution should be exercised when handling instruments with sharp points, or cutting/drilling
CONVENTUS CAGE TM PH Procedure Instruments Instrument Tray Cleaning and Sterilization 1. WARNINGS AND PRECAUTIONS 1.1. Caution should be exercised when handling instruments with sharp points, or cutting/drilling
With Remote Control. English. Model
 Digital Ceramic Heater With Remote Control, Owner s Manual English Model HPQ15F-E TABLE OF CONTENTS 2 INTRODUCTION Thank you for choosing the Hunter Ceramic Heater. This manual gives you complete instructions
Digital Ceramic Heater With Remote Control, Owner s Manual English Model HPQ15F-E TABLE OF CONTENTS 2 INTRODUCTION Thank you for choosing the Hunter Ceramic Heater. This manual gives you complete instructions
Instruction Manual Mini SAD Light Box Code: A54LC
 Instruction Manual Mini SAD Light Box Code: A54LC 1. About the Daylight Instructions for Use Congratulations on the purchase of your new Daylight. The product will offer many years of reliable usage if
Instruction Manual Mini SAD Light Box Code: A54LC 1. About the Daylight Instructions for Use Congratulations on the purchase of your new Daylight. The product will offer many years of reliable usage if
CONVENTUS CAGE TM - DR Procedure Instruments Instrument Tray. Equipment Cleaning and Sterilization
 CONVENTUS CAGE TM - DR Procedure Instruments Instrument Tray Equipment Cleaning and Sterilization Table of Contents 1. WARNINGS AND PRECAUTIONS... 3 2. INSTRUMENT PREPARATION... 3 3. CAVITY PREPARATION
CONVENTUS CAGE TM - DR Procedure Instruments Instrument Tray Equipment Cleaning and Sterilization Table of Contents 1. WARNINGS AND PRECAUTIONS... 3 2. INSTRUMENT PREPARATION... 3 3. CAVITY PREPARATION
TABLE OF CONTENTS. Instructions for Use are updated periodically, so please check willowpump.com for current version.
 TABLE OF CONTENTS 1. Safety Information 2 2. Indications for Use 4 3. How Supplied 4 4. Product Description 4 5. Things to Know 5 Phases of Willow 10 6. Product Parts 13 Cleaning & Maintenance 16 Milk
TABLE OF CONTENTS 1. Safety Information 2 2. Indications for Use 4 3. How Supplied 4 4. Product Description 4 5. Things to Know 5 Phases of Willow 10 6. Product Parts 13 Cleaning & Maintenance 16 Milk
PERSONAL CUEING DEVICE MODEL NO. BDAAU100 INSTRUCTION MANUAL.
 PERSONAL CUEING DEVICE MODEL NO. BDAAU100 INSTRUCTION MANUAL www.agilitas.com.au CONTENTS Device Information 3 Safety Information 3 Features 5 Set up 6 Operation 7 Sensitivity Adjustment 8 Charging 10
PERSONAL CUEING DEVICE MODEL NO. BDAAU100 INSTRUCTION MANUAL www.agilitas.com.au CONTENTS Device Information 3 Safety Information 3 Features 5 Set up 6 Operation 7 Sensitivity Adjustment 8 Charging 10
INSTRUCTIONS FOR OPERATION AND CARE OF
 INSTRUCTIONS FOR OPERATION AND CARE OF LITTLE Dipper Compact Paraffin Bath Model LD-2 800-782-7706 626-968-6681 www.whitehallmfg.com Whitehall Manufacturing is a Member of Acorn Engineering s Family of
INSTRUCTIONS FOR OPERATION AND CARE OF LITTLE Dipper Compact Paraffin Bath Model LD-2 800-782-7706 626-968-6681 www.whitehallmfg.com Whitehall Manufacturing is a Member of Acorn Engineering s Family of
Operator s Manual. WarmTouch. Model WT-5300A Patient Warming System
 Operator s Manual WarmTouch TM Model WT-5300A Patient Warming System To obtain information about a warranty, if any, contact Covidien Technical Services at 1.800.635.5267 or your local representative.
Operator s Manual WarmTouch TM Model WT-5300A Patient Warming System To obtain information about a warranty, if any, contact Covidien Technical Services at 1.800.635.5267 or your local representative.
CLEAN MIST ULTRASONIC HUMIDIFIER. Instruction Manual and Warranty Information READ AND SAVE THESE INSTRUCTIONS IM0040A
 CLEAN MIST ULTRASONIC HUMIDIFIER Instruction Manual and Warranty Information IM0040A READ AND SAVE THESE INSTRUCTIONS Contents Safety Instructions... 1 Unpacking / Specifications... 2 Parts & Contents...
CLEAN MIST ULTRASONIC HUMIDIFIER Instruction Manual and Warranty Information IM0040A READ AND SAVE THESE INSTRUCTIONS Contents Safety Instructions... 1 Unpacking / Specifications... 2 Parts & Contents...
Operator s Manual. Model G17 Disinfection Soak Station for Hysteroscopes, Cystoscopes and ENT scopes
 Model G17 Disinfection Soak Station for Hysteroscopes, Cystoscopes and ENT scopes Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741 Fax: 1-877-329-2482
Model G17 Disinfection Soak Station for Hysteroscopes, Cystoscopes and ENT scopes Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741 Fax: 1-877-329-2482
User Instruction Manual
 SAPPHIRE SERIES 1100 & 1100EC Mattress Replacement System Read Entire Manual Before Operating Device OI-S1100600 Uncontrolled Document Rev 5.0-3/28/2011 ECO031411 User Instruction Manual Sapphire 1100
SAPPHIRE SERIES 1100 & 1100EC Mattress Replacement System Read Entire Manual Before Operating Device OI-S1100600 Uncontrolled Document Rev 5.0-3/28/2011 ECO031411 User Instruction Manual Sapphire 1100
CVX-300 Excimer Laser System. Gen 4.0 Operator s Manual
 CVX-300 Excimer Laser System Gen 4.0 Operator s Manual 0086 This page intentionally left blank. CVX-300 Excimer Laser System Operator's Manual CVX-300 Excimer Laser System ii 2007 THE SPECTRANETICS CORPORATION.
CVX-300 Excimer Laser System Gen 4.0 Operator s Manual 0086 This page intentionally left blank. CVX-300 Excimer Laser System Operator's Manual CVX-300 Excimer Laser System ii 2007 THE SPECTRANETICS CORPORATION.
Operator s Manual. Model G14TC-3 Disinfection Soak Station for Transesophageal Ultrasound Probes
 Model G14TC-3 Disinfection Soak Station for Transesophageal Ultrasound Probes Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741 Fax: 1-877-329-2482
Model G14TC-3 Disinfection Soak Station for Transesophageal Ultrasound Probes Operator s Manual CIVCO Medical Solutions 102 First Street South Kalona, IA 52247 USA Tel: 1-800-445-6741 Fax: 1-877-329-2482
Companion 5. Oxygen Concentrator PROVIDER TECHNICAL MANUAL
 Companion 5 Oxygen Concentrator PROVIDER TECHNICAL MANUAL Table of Contents General Information... 3 Warning and Caution Statements... 3 Introduction to the Companion 5 Oxygen Concentrator... 4 Companion
Companion 5 Oxygen Concentrator PROVIDER TECHNICAL MANUAL Table of Contents General Information... 3 Warning and Caution Statements... 3 Introduction to the Companion 5 Oxygen Concentrator... 4 Companion
With Remote Control. English
 Infrared Heater With Remote Control, Owner s Manual English Model H1500RC-CHY H1500RC-BLK INTRODUCTION Thank you for choosing the Hunter Infrared Heater. This manual gives you complete instructions for
Infrared Heater With Remote Control, Owner s Manual English Model H1500RC-CHY H1500RC-BLK INTRODUCTION Thank you for choosing the Hunter Infrared Heater. This manual gives you complete instructions for
Aluminum Heating Mantles
 Aluminum Heating Mantles ON/OFF HOT User Guide Version 1.3 ON/OFF IEC power inlet socket Power supply switch (large-volume models). Power to either the lower part of the element or the upper and lower
Aluminum Heating Mantles ON/OFF HOT User Guide Version 1.3 ON/OFF IEC power inlet socket Power supply switch (large-volume models). Power to either the lower part of the element or the upper and lower
Patient Warming System Controller Model WC5X Service Manual
 Patient Warming System Controller Model WC5X Service Manual Forward to the Biomedical Engineering Department For information on operating the HotDog Patient Warming System, refer to the User Manual Manufactured
Patient Warming System Controller Model WC5X Service Manual Forward to the Biomedical Engineering Department For information on operating the HotDog Patient Warming System, refer to the User Manual Manufactured
Instructions for Use Spinal Punches IFU
 Indications for use Spinal punches are manually operated instruments indicated for cutting or biting bone during surgery involving the skull or spinal column. Contraindiciations Instruments should not
Indications for use Spinal punches are manually operated instruments indicated for cutting or biting bone during surgery involving the skull or spinal column. Contraindiciations Instruments should not
Instructions for Use
 Instructions for Use IFU-1004-J TABLE OF CONTENTS 1. Safety Information 2 2. Indications for Use 4 3. How Supplied 4 4. Product Description 4 5. Things to Know 5 Phases of Willow 12 6. Product Parts 15
Instructions for Use IFU-1004-J TABLE OF CONTENTS 1. Safety Information 2 2. Indications for Use 4 3. How Supplied 4 4. Product Description 4 5. Things to Know 5 Phases of Willow 12 6. Product Parts 15
AtmosAir Mattress Replacement System User Guide Instructions for Use
 AtmosAir Mattress Replacement System User Guide Instructions for Use AtmosAir with SAT 4000 Series AtmosAir with SAT 9000 Series AtmosAir with SAT APod 25 Series AtmosAir with SAT T-Series AtmosAir with
AtmosAir Mattress Replacement System User Guide Instructions for Use AtmosAir with SAT 4000 Series AtmosAir with SAT 9000 Series AtmosAir with SAT APod 25 Series AtmosAir with SAT T-Series AtmosAir with
Instructions For Use
 Instructions For Use Spectra S1 Plus / S2 Plus PLEASE READ ALL INSTRUCTIONS BEFORE USING THIS PRODUCT MODEL #: SERIAL #: Single Pumping Dual Pumping DO NOT THROW AWAY THIS MANUAL. PLEASE KEEP IT FOR FUTURE
Instructions For Use Spectra S1 Plus / S2 Plus PLEASE READ ALL INSTRUCTIONS BEFORE USING THIS PRODUCT MODEL #: SERIAL #: Single Pumping Dual Pumping DO NOT THROW AWAY THIS MANUAL. PLEASE KEEP IT FOR FUTURE
BL250. Pulse Blender OWNER S GUIDE
 BL250 OWNER S GUIDE Pulse Blender 1-877-646-5288 IMPORTANT SAFETY INSTRUCTIONS For Household Use Only WHEN USING ELECTRICAL APPLIANCES, BASIC SAFETY PRECAUTIONS SHOULD ALWAYS BE FOLLOWED, INCLUDING THE
BL250 OWNER S GUIDE Pulse Blender 1-877-646-5288 IMPORTANT SAFETY INSTRUCTIONS For Household Use Only WHEN USING ELECTRICAL APPLIANCES, BASIC SAFETY PRECAUTIONS SHOULD ALWAYS BE FOLLOWED, INCLUDING THE
Instruction Manual. For All Corning Hot Plates, Stirrers, Stirrer/Hot Plates with Digital Displays, and External Temperature Controller (6795PR)
 Instruction Manual For All Corning Hot Plates, Stirrers, Stirrer/Hot Plates with Digital Displays, and External Temperature Controller (6795PR) Corning Cat. No. 230V 230V Model No. Product Top Plate Size
Instruction Manual For All Corning Hot Plates, Stirrers, Stirrer/Hot Plates with Digital Displays, and External Temperature Controller (6795PR) Corning Cat. No. 230V 230V Model No. Product Top Plate Size
OPERATION & CARE MANUAL FOR P-2010-S BLANKET WARMING CABINET
 OPERATION & CARE MANUAL FOR P-2010-S BLANKET WARMING CABINET BLANKET WARMING CABINET TRANSPORT AND STORAGE Transport and Storage Environmental Conditions (not to exceed 15 days) Ambient temperature range
OPERATION & CARE MANUAL FOR P-2010-S BLANKET WARMING CABINET BLANKET WARMING CABINET TRANSPORT AND STORAGE Transport and Storage Environmental Conditions (not to exceed 15 days) Ambient temperature range
Model IC-1545-DL Multi-Flo DVT Combo
 Bio Compression Systems, Inc. Model IC-1545-DL Multi-Flo DVT Combo Intermittent Pneumatic Compression Device for DVT Prophylaxis U.S. BASED MANUFACTURER Quality Medical Products Since 1983 1 Table of Contents
Bio Compression Systems, Inc. Model IC-1545-DL Multi-Flo DVT Combo Intermittent Pneumatic Compression Device for DVT Prophylaxis U.S. BASED MANUFACTURER Quality Medical Products Since 1983 1 Table of Contents
2364 Leicester Road, P.O. Box 175, Leicester, NY Phone (585) Fax (585)
 Dry Heat Sterilizers with 60 Minute Sterilization Cycle Times MODEL 3100 MODEL 2100 2364 Leicester Road, P.O. Box 175, Leicester, NY 14481 Phone (585) 382-3223 Fax (585) 382-9481 www.cpac.com December
Dry Heat Sterilizers with 60 Minute Sterilization Cycle Times MODEL 3100 MODEL 2100 2364 Leicester Road, P.O. Box 175, Leicester, NY 14481 Phone (585) 382-3223 Fax (585) 382-9481 www.cpac.com December
NPWT PRO and PRO to GO PATIENT USER MANUAL
 NPWT PRO and PRO to GO PATIENT USER MANUAL About Your Cardinal Health NPWT PRO / PRO to GO Your doctor has chosen the Cardinal Health NPWT PRO / PRO to GO device to remove fluid from your wound by using
NPWT PRO and PRO to GO PATIENT USER MANUAL About Your Cardinal Health NPWT PRO / PRO to GO Your doctor has chosen the Cardinal Health NPWT PRO / PRO to GO device to remove fluid from your wound by using
DEHUMIDIFIER. User Manual 50BT, 70BT
 User Manual DEHUMIDIFIER Model 50BT, 70BT Use & Care Guide Please read and follow all safety rules and instructions in this manual before operating. The product warranty is printed on the back of this
User Manual DEHUMIDIFIER Model 50BT, 70BT Use & Care Guide Please read and follow all safety rules and instructions in this manual before operating. The product warranty is printed on the back of this
GoodKnight H 2 O. User's Manual. Heated Humidifier. Ref. : M-146DFU00-20 Revision D
 GoodKnight H 2 O Heated Humidifier User's Manual Ref. : M-146DFU00-20 Revision D Revision D User's Manual Revisions: GoodKnight H 2 O Heated Humidifier The pages below are included in the American version
GoodKnight H 2 O Heated Humidifier User's Manual Ref. : M-146DFU00-20 Revision D Revision D User's Manual Revisions: GoodKnight H 2 O Heated Humidifier The pages below are included in the American version
INSTRUCTIONS FOR OPERATION AND CARE OF
 INSTRUCTIONS FOR OPERATION AND CARE OF LITTLE Champ Compact Whirlpool AW-606 & AW-607 800-782-7706 626-968-6681 www.whitehallmfg.com Member of 6900-193-001 July 2017 Introduction...3 Model Descriptions...
INSTRUCTIONS FOR OPERATION AND CARE OF LITTLE Champ Compact Whirlpool AW-606 & AW-607 800-782-7706 626-968-6681 www.whitehallmfg.com Member of 6900-193-001 July 2017 Introduction...3 Model Descriptions...
WINIX AIR PURIFIER. User Manual. Tower XQ
 User Manual WINIX AIR PURIFIER Model Tower XQ Use & Care Guide Please read and follow all safety rules and instructions in this manual before operating. The product warranty is printed on the back of this
User Manual WINIX AIR PURIFIER Model Tower XQ Use & Care Guide Please read and follow all safety rules and instructions in this manual before operating. The product warranty is printed on the back of this
CLEANING INSTRUCTIONS
 Reusable Instrument Care Manual Pagoda Pedicle Screw System Manufacturer Ortho Development Corporation 12187 South Business Park Drive Draper, UT 84020 Phone 801-553-9991 Fax 801-553-9993 www.orthodevelopment.com
Reusable Instrument Care Manual Pagoda Pedicle Screw System Manufacturer Ortho Development Corporation 12187 South Business Park Drive Draper, UT 84020 Phone 801-553-9991 Fax 801-553-9993 www.orthodevelopment.com
Here is what comes in your box:
 Here is what comes in your box: We recommend that you pull everything out of the box and lay it out. We have grouped the drawn components below with the hardware you ll need for those parts. The screws
Here is what comes in your box: We recommend that you pull everything out of the box and lay it out. We have grouped the drawn components below with the hardware you ll need for those parts. The screws
Ultrasonic Cool Mist Humidifier
 Model No.: LV450CH Ultrasonic Cool Mist Humidifier Questions or or Concerns? Please contact us Monday Mon-Fri 9:00AM-5:00PM - Friday am - PST 5:00 pm PST at support@levoit.com (888) 726-8520 email or at
Model No.: LV450CH Ultrasonic Cool Mist Humidifier Questions or or Concerns? Please contact us Monday Mon-Fri 9:00AM-5:00PM - Friday am - PST 5:00 pm PST at support@levoit.com (888) 726-8520 email or at
CLEAN MIST SMART HUMIDIFIER Instruction Manual and Warranty Information IM0031B READ AND SAVE THESE INSTRUCTIONS
 CLEAN MIST SMART HUMIDIFIER Instruction Manual and Warranty Information IM0031B READ AND SAVE THESE INSTRUCTIONS Contents Safety Instructions... 1 Unpacking / Specifications... 2 Parts & Contents... 3
CLEAN MIST SMART HUMIDIFIER Instruction Manual and Warranty Information IM0031B READ AND SAVE THESE INSTRUCTIONS Contents Safety Instructions... 1 Unpacking / Specifications... 2 Parts & Contents... 3
AS Medizintechnik GmbH Sattlerstrasse 15, Tuttlingen, Germany Tel 07461/ Fax 07461/
 General Information Use The Air drill System is a pneumatic powered system used for many applications orthopedic and trauma surgery. To ensure proper operation of the air drill, use only original attachment
General Information Use The Air drill System is a pneumatic powered system used for many applications orthopedic and trauma surgery. To ensure proper operation of the air drill, use only original attachment
User Guide for Reprocessing of the SUPER VIEW Wide Angle Viewing System Sterilizable Components and Accessories
 User Guide for Reprocessing of the SUPER VIEW Wide Angle Viewing System Sterilizable Components and Accessories Table of Contents 1. Intended Use Statement... 3 2. Proper Use... 3 3. General Information...
User Guide for Reprocessing of the SUPER VIEW Wide Angle Viewing System Sterilizable Components and Accessories Table of Contents 1. Intended Use Statement... 3 2. Proper Use... 3 3. General Information...
(The chances are you re never going to read me) Owner s Manual.
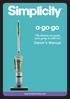 (The chances are you re never going to read me) Owner s Manual www.simplicityvac.com . CONTENTS Getting Started Important Safety Instructions 2 Polarization Instructions 3 State of California Proposition
(The chances are you re never going to read me) Owner s Manual www.simplicityvac.com . CONTENTS Getting Started Important Safety Instructions 2 Polarization Instructions 3 State of California Proposition
SeedVac TM REF 90091
 WWW..COM SeedVac TM REF 90091 STANDARD IMAGING INC. 7601 Murphy Drive Middleton, WI 53562 Feb / 2005 2005 Standard Imaging Inc. DOC #80191-06 TEL 800.261.4446 TEL 608.831.0025 FAX 608.831.2202 www.standardimaging.com
WWW..COM SeedVac TM REF 90091 STANDARD IMAGING INC. 7601 Murphy Drive Middleton, WI 53562 Feb / 2005 2005 Standard Imaging Inc. DOC #80191-06 TEL 800.261.4446 TEL 608.831.0025 FAX 608.831.2202 www.standardimaging.com
User Manual. Mistral-Air Plus Warming Unit. MA1100-EU ( V~, 50/60 Hz) MA1100-US ( V~, 60 Hz) MA1100-JP ( V~, 50/60 Hz)
 EN User Manual Mistral-Air Plus Warming Unit MA1100-EU (220-240V~, 50/60 Hz) MA1100-US (110-120V~, 60 Hz) MA1100-JP (100-110V~, 50/60 Hz) Foreword... 3 Disclaimer... 3 1 Contra-indications, Safety Precautions,
EN User Manual Mistral-Air Plus Warming Unit MA1100-EU (220-240V~, 50/60 Hz) MA1100-US (110-120V~, 60 Hz) MA1100-JP (100-110V~, 50/60 Hz) Foreword... 3 Disclaimer... 3 1 Contra-indications, Safety Precautions,
Important user information
 USER S MANUAL Important user information All users must read this entire manual to fully understand the safe use of EMMA. Declaration of conformity 0413 Complies with 93/42/EEC Medical Device Directive.
USER S MANUAL Important user information All users must read this entire manual to fully understand the safe use of EMMA. Declaration of conformity 0413 Complies with 93/42/EEC Medical Device Directive.
Operator s Manual. WarmTouch. Model WT-5900 Patient Warming System
 Operator s Manual WarmTouch TM Model WT-5900 Patient Warming System To obtain information about a warranty, if any, contact Covidien Technical Services at 1.800.635.5267 or your local representative. Purchase
Operator s Manual WarmTouch TM Model WT-5900 Patient Warming System To obtain information about a warranty, if any, contact Covidien Technical Services at 1.800.635.5267 or your local representative. Purchase
