Trade of Plumbing. Module 3: Domestic Heating/MMA Welding Unit 3: Domestic Heating System Installation Phase 2
|
|
|
- Randell Green
- 5 years ago
- Views:
Transcription
1 Trade of Plumbing Module 3: Domestic Heating/MMA Welding Unit 3: Domestic Heating System Installation Phase 2
2
3 Table of Contents List of Figures... 5 List of Tables... 6 Document Release History... 7 Module 3 Domestic Heating/MMA Welding... 8 Unit 3 Domestic Heating System Installation... 8 Learning Outcome:... 8 Key Learning Points:... 8 Training Resources:... 9 Exercise... 9 Key Learning Points Code... 9 Thermal Expansion Coefficient of Thermal Expansion Thermal Insulation Materials and their Applications Properties of Insulating Materials Thermal Insulating Materials For Pipes, Storage Vessels, etc James Joule Specific Heat James Watt Power Heat Transfer Conduction Convection Radiation Conduction Thermal Conductivity of Materials Methods of Heat Transfer Self Assessment Specific Heat Questions Specific Heat Questions continued Power Questions Power Questions continued Unit 3 3
4 Index Unit 3 4
5 List of Figures Figure 1. Thermal Insulation Figure 2. Insulation of Cistern, Lid, and Pipes Figure 3. James Prescott Joule Figure 4. Rates of Heat Flow Figure 5. Methods of Heat Transfer Unit 3 5
6 List of Tables Table 1. Coefficients of Thermal Expansion Table 2. Insulating types and application Table 3. Specific Heat Unit 3 6
7 Document Release History Date Version Comments June 2006 V /02/ SOLAS transfer Unit 3 7
8 Module 3 Domestic Heating/MMA Welding Unit 3 Domestic Heating System Installation Duration 35 Hours Learning Outcome: By the end of this unit each apprentice will be able to: State the various types of boilers and heating controls. Calculate thermal expansion in plumbing materials. Calculate specific heat capacities of liquids and solids. Calculate the kilowatt rating of boilers. Install a basic domestic heating system project using copper, pex, P.B. pipe. Calculate the cost of installation of a domestic heating system. Key Learning Points: Rk Rk Rk Sc M Rk Sc M Types of boilers. Domestic heating controls, heating zones, basic electricity. Flues. Thermal expansion, linear coefficients of expansion, expansion loops. Thermal insulation. Specific heat. Rk M Radiator and boiler sizing. Sk Sk Sk H P P P P P Interpretation of drawings. Installation of heating system project fitting boiler, cylinder, radiators, feed and expansion tank, pipework and fittings etc. Use of hand tools, power tools, gas torches. Good working practice. Initiative. Planning. Teamwork. Communication. Unit 3 8
9 Sk M Pricing and estimating. Training Resources: Classroom facilities. Information sheets. Workshop facilities. Sample heating controls. Exercise Two apprentices to install a basic domestic heating system project from schematic Drawing No in the curriculum document. Key Learning Points Code M = Maths D= Drawing RK = Related Knowledge Sc = Science P = Personal Skills Sk = Skill H = Hazards Unit 3 9
10 Thermal Expansion It is a well known fact that when a substance or material is heated it gets larger or expands; and when it cools it gets smaller or contracts. In other words, material moves as its temperature varies, and this is called thermal expansion. Why do materials expand when heated and contract on cooling? Imagine the space taken up by a group of people standing fairly still. Now, if all these people started to rock-n-roll, they would jostle and push one another about, and in doing so they would take up more space. The separate molecules which go to make up a material are always on the move, vibrating to and fro at a rate depending upon the amount of heat energy they possess. When cold, their heat energy is small and they keep fairly still and close to one another, taking up as little space as possible. When the material is heated, its molecules gain energy and start to dance with increasing vigour and so, of course, they take up more space. The result is an increase in the size of the material; that is, the material expands on being heated. When the material cools down it loses heat energy, the molecules slow down, and a reduction in size or contraction results. Coefficient of Thermal Expansion The amount any material will expand when it is heated depends upon the material itself; whether it is in a solid, liquid or gaseous state; and upon its heat content. The amount that solids will expand for each ºC of temperature rise is easily measured and is fairly constant. To whatever extent a solid expands or contracts for 1º change in temperature, it will expand or contract ten times as much for 10º change in temperature. Liquids and gases do not behave quite so conveniently, and water in particular behaves in a most unexpected manner. For the present, consider the effect of thermal movement on solid materials such as pipes, boilers and sheet metal roof coverings. This thermal movement affects the material in all its dimensions. The length, width and thickness all increase as the temperature of the material increases. However, since most of the materials the plumber deals with, e.g. pipes and bays of sheet metal on roofs, are so much longer than they are wide or thick, it will only be necessary to examine the more readily seen effects of heat on the length of the material. To calculate the physical amount a material will expand or contract, reference will be made to the LINEAR COEFFICIENTS OF THERMAL EXPANSION. The word linear means lengthwise and coefficient means fraction, so the term really describes that fraction by which a given or unit length of material will expand when its temperature is increased by 1º, or will contract when its temperature decreases by 1º. Unit 3 10
11 If a piece of material 1m long expands by 1mm when its temperature is raised by 1ºC, it can be said to have expanded by one thousandth of its length. This number written as a decimal would read and relates to the expansion of that material per degree Celsius temperature rise. This fraction is referred to as the coefficient of linear expansion. To calculate the increase in length of a given piece of material due to thermal expansion, three pieces of information must be known. (i) The original length of the material; (ii) The increase in temperature by which it is raised; (iii) The coefficient of expansion for that material. The coefficient of thermal expansion rates of use to the plumber are given in the following table: Table 1. Coefficients of Thermal Expansion MATERIAL APPROXIMATE COEFFICIENT PER ºC Copper Mild Steel Plastic Lead Aluminium The calculation used to find the amount material expands in length (linear expansion) is expressed as: Length (in mm) X temperature change X Coefficient Example 1 Find the amount a 9m long plastic pipe will expand due to a temperature rise of 24ºC. = 9,000 X 24 X = 38.9mm This emphasises the need to allow for expansion when using plastic pipes. Unit 3 11
12 Thermal Insulation Thermal insulation may be described as the application of special materials to pipes and storage vessels to prevent excessive loss of heat energy and damage from frost. Insulation material entraps small pockets of air, since still air is a bad conductor of heat. Apart from trapping pocks of air a good insulation material should not be flammable and if used externally or in damp conditions, sufficient precautions should be taken to ensure that the material is kept draught-proof and impervious to moisture. A good insulation material should have a low thermal conductivity. This means that it must resist the passage of heat through it. Porosity is another property that insulation should have. The value of still air as a thermal insulator has already been mentioned. If you could wrap a layer of really still air around a pipe you would do a good job of insulation. This is impossible, of course, but a similar effect can be created by using good insulation material. Most of the modern insulating materials are sponge-like or fibrous in structure, containing millions of tiny air cells which trap air and hold it still. Good insulation materials should be resistant to vermin and fungal attack. Materials such as glass fibre, mineral wood, foamed plastics and polystyrene are excellent choices and are used extensively throughout the building industry giving excellent results. In industrial installations thermal insulation is usually fitted by specialist contractors. However, in domestic work this task is often carried out by the plumber. Careful attention should be given to this job and coverage must be complete and uniform if it is to be fully effective. Gaps between sections and skimped thickness must always be avoided. Figure 1. Thermal Insulation Unit 3 12
13 Materials and their Applications Glass Fibre Used in loose fill form to insulate wall cavities and attic spaces. Also available in blanket or roll form. Foam Rubber Available in two metre lengths and used to insulate pipes. Polystyrene Available in sheet form and often used to insulate storage cisterns in roof spaces. Purpose made insulating jackets in many sizes are also made for hot water storage cylinders. They are made in a wide variety of materials, such as fibre glass, mineral wool, and sheep s wool treated against vermin and in covers ranging from brown calico to washable plastics. The fuel savings effected with one of these jackets can be quite considerable and it is a fact that in most cases the cost of insulation pays for itself in a year or two, an investment well worth making. Properties of Insulating Materials 1. Low Thermal Conductivity. 2. Incombustible. 3. Moisture Resistant. 4. Porosity. 5. Resistant to Vermin. 6. Resistant to Fungal Attack. 7. Weight. 8. Ease of Application. 9. A Good Surface Finish. Unit 3 13
14 Figure 2. Insulation of Cistern, Lid, and Pipes Thermal Insulating Materials For Pipes, Storage Vessels, etc. TYPE Table 2. Loose fill (in granular or fibrous form) for example mica-fil and fibre-glass. Insulating types and application APPLICATION Infill to preformed cavities; for example, between hot or cold store vessels and or their prepared casings, round pipes and so on in chases or casings. Flexible (in strip or blanket form). RIGID (in preformed ready-to-fit sections). Wrap around covering for curved surfaces such as pipes, more especially for hot store cylinders, and possibly for rectangular store cisterns and hot water tanks. Made specially for pipes. Also in flat sections for hot water tank and cold store cisterns lagging sets. PLASTIC (that is workable until set). For covering awkward or irregular shapes such as uncased boiler surfaces, large cylinders and valves. Unit 3 14
15 James Joule Figure 3. James Prescott Joule James Prescott Joule s family owned a brewery and, as a young man, he was independently wealthy. He used his money to perform experimental research in several areas. His greatest asset was the ability to think precisely. His work showed his inescapable logic. All of his research began as an attempt to improve the efficiency of the electric motor so it could replace the steam engine in industrial applications. Joule decided to measure the heat developed by electric currents. He accurately determined the mechanical equivalent of heat, thus verifying the conservation of energy. But the science community was slow in accepting his results, perhaps because they didn t understand the implications. Later in life, he worked with William Thomson (Lord Kelvin). Among other things, Joule invented arc welding and the displacement pump. Finally in 1875, his money from conducting experiments ran out. Years of illness followed ending with his death in Unit 3 15
16 Specific Heat Specific heat is the amount of heat required to raise 1kg of material 1ºC. The specific heat of water is 4.186kJ / kg deg C. The heat required differs from material to material as indicated by the table below. Table 3. Specific Heat MATERIAL KILOJOULES / KILOGRAM DEG C Water Aluminium Cast Iron Mild Steel Copper Lead A knowledge of specific heats, and the Heat Energy requirements to raise a given mass of substance through a given rise in temperature is necessary in work on domestic hot water and central heating systems. One or two examples will show how easy it is to do these calculations. Example 1 How many kilojoules will be required to raise 75 litres of water from 12ºC to 55ºC? Heat energy required = Mass X Sp Heat X Rise in Temperature (kj) = (kg) (kj/kg) (deg C) = 75 X X 43 Answer = 13, kj Unit 3 16
17 James Watt James Watt was born in Greenock, Scotland on January 19 th His father was the local magistrate who also owned a ship building and house building business. As a child he was quite delicate and sickly and did not attend school. Instead his mother taught him at home. He also spent many hours in his father s workshop making models usually of cranes and bridges. At the age of 17 he decided to become a mathematical instrument maker and left home to take up his studies in his area. By 1763 he had returned to Scotland where he set himself up as an instrument maker in a university. A year later he married his cousin, who bore him 6 children before her death 9 years later. In 1764, while repairing a model steam engine, he noticed that it wasted a considerable amount of steam. To improve the engine s efficiency, he invented a condensing chamber where the steam could condense to hot water before being re-heated in the engine and turned back to steam again. Although he took out a patent on his condensing chamber, he did not have the necessary finance to build a full scale engine. In 1766 he became a land surveyor, and spent the next 8 years marking out routes for canals in Scotland. In 1775 he went into partnership with Mathew Boulton, who was aware of Watt s patent and was keen to put his invention into production. Their partnership was to last 25 years. A year later Watt married again. The years that followed were to be the most productive in Watt s life. He built many engines mainly for the copper and tin mining industry. In 1782 Watt designed what many people think was his greatest invention the double acting piston which pushed as well as pulled in parallel motion. Even today, over two hundred years later, this design is still used in the combustion engine of motor cars. Demands for this new engine came pouring in to Watt & Boulton, mainly from paper or flour mills, distilleries, canals and waterworks. By 1790 Watt had become a very wealthy man accruing over 76,000 from royalties alone. In 1800 he retired but continued with his inventions. One of these was a sculpturing machine which he use to reproduce original busts and figures for his friends. During the remaining years of his life Watt received many honours from the science community but refused to take the title of Baronetcy from King George III. James Watt died on August 1819 at the age of 83. Seldom can the achievement of one man be said to have affected the progress and lives of millions of people for hundreds of years. But such is the contribution of Watt. Indeed, many people believe the industrial revolution might never have happened if not for him. Unit 3 17
18 Power The SI unit of power is the watt. 1,000 watts = 1 kilowatt (KW) The joule, which is a measure of heat energy = 1 watt per second. or J = w/s Similarly kj = kw / S The answer in the previous examples give the amount of heat energy required if the substances involved were to be heated through the stated temperature rise in ONE SECOND. Clearly this is impractical, because in practice the heating up periods would be much longer. To express the power required in one hour simply divide the answer by 3,600, which is the number of seconds in one hour, or 7,200 for two hours and so on. In example one, 13,499 kj of heat energy was required to heat 75 litres of water from 12ºC to 55ºC in one second. If this was to be achieved in 1 hour the formula would be: 13,499 = 3.75 kw 3,600 If it was to be achieved in 3 hours the formulas would be: 13,499 = 13,499 = 1.25 kw 3,600 x 3 10,800 Question 150 How many joules are required to raise 45 litres of water from 10ºC to 60ºC? Show all working (1966 Examination) Answer 4.2 kj (kilojoules) will raise 1 litre water 1 degree C so kj = 4.2 X 45 X (60ºC - 10ºC) = 9450 then Joules = 9.450,000 Note 1 kj = 1000 J Question 151 If the domestic hot water load is 9,450,000 joules, calculate the power in kilowatts to perform this duty in one hour. Show all working Answer 9,450,000 joules = 9450 kj 1 kilowatt (kw) = 1 kj/s Unit 3 18
19 or Power (kw) = Energy (kj) Time (s) = 9450 kilojoules 3600 seconds in 1 hour = 2.62 kw If 55 litres of water is to be heated from 8ºC to 70ºC, in half an hour what size heater would be required. Power (kw) = Mass X Sp Heat X Temperature Time in seconds = 55 X X = = 7.93 kw (8 kw) 1800 = = 3.96 (4 kw) 3600 Unit 3 19
20 Heat Transfer Some knowledge of the ways in which heat is transferred is necessary to understand fully the working principles of central heating and hot water systems. There are three methods of heat transfer conduction, convection and radiation. Each of these will be discussed separately. Conduction Conduction is the transfer of heat through or along a solid. If you hold a metal rod and heat up one end with a blowtorch the other end would soon become warm. This is because the heat is being transferred through the metal. Heat travels through all materials but the speed at which it travels varies. The faster the heat travels the better the material is at conduction. Convection Convection is a form of heat transmission peculiar to liquids and gases. Water and air are typical materials in which it occurs. Very briefly, it may be described as the transmission of heat by the actual movement of particles of liquid or gas. This movement is caused by the change in the particles weight brought about by a variation in their temperature. This form of heat transfer explains the movement of heated gases up a fuel pipe or chimney; the movement of water through the circulatory pipework of a hot-water system; and the movement of heated water around the pipework and radiators of a central heating system. It also explains the movement of warmed air around a room. Radiation Radiation is the transfer of heat energy in the form of straight lines. Radiant heat will pass through the air without appreciably warming it. The heat from the sun is a good example of radiant heat as it travels through millions of miles of space to reach the earth. This heat will also pass through the air without appreciably warming it, but any solid object obstructing the rays will become warmed by them. The rate at which a surface absorbs heat depends upon its colour. A blackened surface is an excellent absorber of heat as well as an excellent emitter. Objects that are good absorbers of heat are also good emitters. A surface that is painted silver will not absorb or emit heat readily. Unit 3 20
21 Figure 4. Rates of Heat Flow Unit 3 21
22 Conduction The transference of heat through or along a solid Thermal Conductivity of Materials Copper Aluminium Iron Glass Brick Water Wood Still Air GOOD CONDUCTORS BAD CONDUCTORS Convection The transference of heat through a Liquid or a Gas. Example Liquid: The water in a hot water cylinder is heated from the boiler below by convection currents. Gas: Smoke from a fire is carried up the chimney by convection currents. Radiation The transference of heat from its source to another solid object through Air or Space. Some Examples From the Sun to You. From a Fire to You. Unit 3 22
23 Methods of Heat Transfer Figure 5. Methods of Heat Transfer Unit 3 23
24 Self Assessment Specific Heat Questions Exercise 1 Find the amount of kilojoules to raise the temperature of 150 litres of water from 10ºC to 60ºC. Exercise 2 Calculate the quantity of heat in kj required to raise the temperature of 10 litres of water from 12ºC to 50ºC. Exercise 3 Calculate the quantity of heat in kj given off when 200 litres of water cools from 90ºC to 15ºC. Exercise 4 Calculate the quality of heat in Mega Joules required to raise the temperature of 1,500 litres of water from 10ºC to 80ºC. Note: 1,000 kilojoules (kj) = 1 Mega Joule (MJ) Answers to Exercise 1 to 4 are on the next page. Unit 3 24
25 Exercise 1 Answer Heat energy required = Mass X Sp Heat X Rise in Temperature = 150 X X 50 = 31,395 kj Exercise 2 Answer Heat energy required = Mass X Sp Heat X Rise in Temperature = 10 X X 38 = 1,590 kj Exercise 3 Answer Heat energy required = Mass X Sp Heat X Rise in Temperature = 200 X X 75 = 62,790 kj Exercise 4 Answer Heat energy required = Mass X Sp Heat X Rise in Temperature = 1,500 X X 70 = 439,530 kj = 439,530 1,000 = MJ Specific Heat Questions continued 1. How many kilojoules are required to raise 45 litres of water from 10ºC to 60ºC? 2. Calculate the number of joules required to raise 140 litres of water from 12ºC to 100ºC. 3. Calculate the quantity of heat energy required to raise the temperature of 100 litres of water from 20ºC to 85ºC. 4. If a billet of aluminium 25kg in weight is heated from 9ºC to 90ºC how many kilojoules of heat energy will be required for this operation? Also show your answer in joules. 5. How many joules are required to raise litres of water from 1ºC to 75.75ºC? 6. If 560kg of lead is heated through 255ºC. How much heat energy would be required? 7. If 1 tonne of copper is heated from 15ºC to 555ºC, how much heat energy is required? Give your answer in megajoules (MJ). 8. If 1.75 tonnes of water is heated through 45.50C, calculate the heat energy requirements for this operation. 9. A rectangular hot water storage vessel measures 1.25 metres long, 1255mm wide and 555mm high. If it is filled with water, calculate the amount of heat energy required to raise its temperature from 10ºC to 87ºC. 10. A hot water storage cylinder is 1.25 metres high and 55mm in diameter. How many kilojoules of heat energy are required to raise the water temperature from 13ºC to 95ºC? Unit 3 25
26 Power Questions Exercise 1 Calculate the boiler power in kw required to heat 140 litres of water through 55ºC in one hour. Exercise 2 Find the size in kw of an electric water heater capable of heating 45 litres of water from 15ºC to 55ºC in one hour. Exercise 3 A gas fired boiler is required to raise 155 litres of water from 12ºC to 61ºC in three hours. Calculate the size of the boiler. Answers are on the next page. Unit 3 26
27 Exercise 1 Answer Mass X Specific Heat X Temperature Rise Time in seconds 140 X X 55 3,600 = 8.95 kw Exercise 2 Answer Mass X Specific Heat X temperature Rise Time in seconds 45 X X 40 3,600 = 2.09 kw Exercise 3 Answer Mass X Specific Heat X Temperature Rise Time in seconds 155 X X 49 3,600 X X X 49 10,800 = 2.94 kw Power Questions continued 1. Calculate the boiler power in kilowatts required to heat 159 litres of water through in one hour. 2. Calculate the power in kw required to raise 45kg of water from 15ºC to 70ºC in 2 hours. 3. Find the power required to heat 136 litres of water in one hour from 6ºC to 60ºC. 4. An electric water heater is required to heat 55 litres of water from 9ºC to 75ºC in 30 minutes.calculate the size of the heater. 5. A central heating system holds 350 litres of water. If the water in the system is to be heated through 72ºC in 35 minutes what size boiler would be required? 6. What size gas boiler would be required to heat 100 litres of water from 6ºC to 92ºC in 1 hour 15 minutes? 7. A billet of aluminium weighs 45kg. Calculate the power required to heat it through 660ºC in 2 hours 20 minutes. Specific heat capacity of aluminium = Calculate the power in kw required to heat 140 litres of water from 12ºC to 67ºC in one hour. Unit 3 27
28 9. One tonne of lead is to be heated from 100ºC to 327ºC in 18 minutes. Calculate the power required to carry out this operation. Specific heat capacity of lead = A hot water storage cylinder has a capacity of 136 litres. Calculate the time in hours it will take to raise the temperature of the water from 10ºC to 60ºC by means of a 3kW immersion heater. Unit 3 28
29 Index C conduction... 20, 22 conductors bad good convection... 20, 22 F foam rubber G glass fibre H heat unit of heat transfer methods of I insulating jackets J James Joule James Watt P polystyrene porosity... 12, 13 power unit of R radiation... 20, 22 S specific heat T thermal expansion coefficient of linear coefficients of thermal insulating materials properties of thermal insulation Unit 3 29
Trade of Plumbing. Module 2: Domestic Hot and Cold Water Service Unit 10: Hot Water Supply Phase 2
 Trade of Plumbing Module 2: Domestic Hot and Cold Water Service Unit 10: Hot Water Supply Phase 2 Table of Contents List of Figures... 4 List of Tables... 5 Document Release History... 6 Module 2 Domestic
Trade of Plumbing Module 2: Domestic Hot and Cold Water Service Unit 10: Hot Water Supply Phase 2 Table of Contents List of Figures... 4 List of Tables... 5 Document Release History... 6 Module 2 Domestic
Q1. The diagram shows an experiment to find out what happens to infrared waves when they strike different surfaces.
 Q1. The diagram shows an experiment to find out what happens to infrared waves when they strike different surfaces. (a) The water in the black tube gets hotter than the water in the shiny tube. Choose
Q1. The diagram shows an experiment to find out what happens to infrared waves when they strike different surfaces. (a) The water in the black tube gets hotter than the water in the shiny tube. Choose
Different energy sources can be used to generate electricity.
 Q1. Electricity is a useful form of energy. (a) Different energy sources can be used to generate electricity. Give one advantage and one disadvantage (other than cost) of using each energy source to generate
Q1. Electricity is a useful form of energy. (a) Different energy sources can be used to generate electricity. Give one advantage and one disadvantage (other than cost) of using each energy source to generate
(a) (i) Through which part of the house is most heat lost? How can the heat loss through the windows be reduced? ...
 Q1. The diagram shows where heat is lost from a house that is not insulated. (a) (i) Through which part of the house is most heat lost? (ii) How can the heat loss through the windows be reduced? (b) A
Q1. The diagram shows where heat is lost from a house that is not insulated. (a) (i) Through which part of the house is most heat lost? (ii) How can the heat loss through the windows be reduced? (b) A
Describe the movement of the particles of helium gas inside the balloon (2)
 The figure below shows a balloon filled with helium gas. (a) Describe the movement of the particles of helium gas inside the balloon............. (2) (b) What name is given to the total kinetic energy
The figure below shows a balloon filled with helium gas. (a) Describe the movement of the particles of helium gas inside the balloon............. (2) (b) What name is given to the total kinetic energy
Unit THE NATURE OF HEAT
 Unit 5.0 - THE NATURE OF HEAT Heat is a form of energy, in the form of infrared radiation. Heat from the sun travels through space at the speed of 300,000,000 m/s. Upon arriving on earth, much of the radiant
Unit 5.0 - THE NATURE OF HEAT Heat is a form of energy, in the form of infrared radiation. Heat from the sun travels through space at the speed of 300,000,000 m/s. Upon arriving on earth, much of the radiant
A student investigated how much energy from the Sun was incident on the Earth s surface at her location.
 A student investigated how much energy from the Sun was incident on the Earth s surface at her location. She put an insulated pan of water in direct sunlight and measured the time it took for the temperature
A student investigated how much energy from the Sun was incident on the Earth s surface at her location. She put an insulated pan of water in direct sunlight and measured the time it took for the temperature
(a) (i) Complete the following sentences using words from the box. The warm air rising from the heater transfers energy to the
 Q1.The diagram shows how one type of electric storage heater is constructed. The heater has ceramic bricks inside. The electric elements heat the ceramic bricks during the night. Later, during the daytime,
Q1.The diagram shows how one type of electric storage heater is constructed. The heater has ceramic bricks inside. The electric elements heat the ceramic bricks during the night. Later, during the daytime,
Section 9. Comparing Energy Consumption: More for Your Money. What Do You See? What Do You Think? Investigate. Learning Outcomes
 Section 9 Comparing Energy Consumption: More for Your Money Section 9 Comparing Energy Consumption: More for Your Money What Do You See? Learning Outcomes In this section, you will Measure and compare
Section 9 Comparing Energy Consumption: More for Your Money Section 9 Comparing Energy Consumption: More for Your Money What Do You See? Learning Outcomes In this section, you will Measure and compare
When both switches are on, the heater works at the high power setting. What is the power of the heater when it is switched to the high power setting?
 1 (a) The diagram shows two switches on a room heater. The heater has three power settings. The power produced by two of the settings is given in the table. Setting Power in kw Low 0.5 Medium 1.5 High
1 (a) The diagram shows two switches on a room heater. The heater has three power settings. The power produced by two of the settings is given in the table. Setting Power in kw Low 0.5 Medium 1.5 High
Use each of the terms in the box to explain how heat is lost from inside a house through the window. conduction convection radiation
 Q1. The diagram shows a side view of a double-glazed window. (a) Use each of the terms in the box to explain how heat is lost from inside a house through the window. conduction convection radiation..................
Q1. The diagram shows a side view of a double-glazed window. (a) Use each of the terms in the box to explain how heat is lost from inside a house through the window. conduction convection radiation..................
Q1. The table gives information about some methods of conserving energy in a house.
 Q1. The table gives information about some methods of conserving energy in a house. Conservation method Installation cost in Annual saving on energy bills in Cavity wall insulation 500 60 Hot water tank
Q1. The table gives information about some methods of conserving energy in a house. Conservation method Installation cost in Annual saving on energy bills in Cavity wall insulation 500 60 Hot water tank
Fundamentals of Heat Transfer
 Fundamentals of Heat Transfer State basic laws of heat transfer Estimate heat transfer rates by different modes Use the concept of thermal resistance to solve steady state heat transfer problems State
Fundamentals of Heat Transfer State basic laws of heat transfer Estimate heat transfer rates by different modes Use the concept of thermal resistance to solve steady state heat transfer problems State
People living in the desert need to wear special clothing in order for them to keep cool.
 Heat Keep Your Cool Humans live in a number of different places, some of which can be very hot or very cold. Inuit people can live in very cold climates, because their clothing and homes help to keep them
Heat Keep Your Cool Humans live in a number of different places, some of which can be very hot or very cold. Inuit people can live in very cold climates, because their clothing and homes help to keep them
Thermal Energy. Conduction, Convection, and Radiation. Before You Read. Read to Learn. Conduction. section 2
 chapter 5 Thermal Energy section 2 Conduction, Convection, and What You ll Learn the three ways heat is transferred the difference between insulators and conductors how insulators control the transfer
chapter 5 Thermal Energy section 2 Conduction, Convection, and What You ll Learn the three ways heat is transferred the difference between insulators and conductors how insulators control the transfer
The table gives information about some ways of reducing the energy consumption in a house. Installation cost in. Fit a new hot water boiler
 ## (a) The table gives information about some ways of reducing the energy consumption in a house. Method of reducing energy consumption Installation cost in Annual saving on energy bills in Fit a new hot
## (a) The table gives information about some ways of reducing the energy consumption in a house. Method of reducing energy consumption Installation cost in Annual saving on energy bills in Fit a new hot
Temperature & Heat Heat is a type of energy. It is measured in joules (J).
 Temperature & Heat Heat is a type of energy. It is measured in joules (J). Temperature is a measure of how hot or cold something is. It is measured in degrees Celsius ( C). Heat energy travels from hot
Temperature & Heat Heat is a type of energy. It is measured in joules (J). Temperature is a measure of how hot or cold something is. It is measured in degrees Celsius ( C). Heat energy travels from hot
Changes of State. Lesson 1
 Lesson 1 Changes of State If all the ice in the world melted, the oceans would rise by more than 65 meters (215 feet)! This iceberg is melting in Paraiso Bay, Antarctica. What happens to ice when it melts?
Lesson 1 Changes of State If all the ice in the world melted, the oceans would rise by more than 65 meters (215 feet)! This iceberg is melting in Paraiso Bay, Antarctica. What happens to ice when it melts?
Well Insulated Houses: Helping to Stay Warm in Winter and Cool in Summer
 CON EDISON WEB-BASED MIDDLE SCHOOL ACTIVITY Well Insulated Houses: Helping to Stay Warm in Winter and Cool in Summer Overview In this activity, you and your students will build two house models from discarded
CON EDISON WEB-BASED MIDDLE SCHOOL ACTIVITY Well Insulated Houses: Helping to Stay Warm in Winter and Cool in Summer Overview In this activity, you and your students will build two house models from discarded
PHYSICS OF FOIL HEAT GAIN/LOSS IN BUILDINGS
 PHYSICS OF FOIL HEAT GAIN/LOSS IN BUILDINGS There are three modes of heat transfer: CONDUCTION, CONVECTION, and RADIATION (INFRA-RED). Of the three, radiation is the primary mode; conduction and convection
PHYSICS OF FOIL HEAT GAIN/LOSS IN BUILDINGS There are three modes of heat transfer: CONDUCTION, CONVECTION, and RADIATION (INFRA-RED). Of the three, radiation is the primary mode; conduction and convection
ISLAMAYA ENGLISH SCHOOL
 1 (a) Some water is poured onto a plastic table-top, forming a puddle. The same volume of water is poured into a plastic dish, which is placed alongside the puddle. This is illustrated in Fig. 7.1. water
1 (a) Some water is poured onto a plastic table-top, forming a puddle. The same volume of water is poured into a plastic dish, which is placed alongside the puddle. This is illustrated in Fig. 7.1. water
Heat Transfer. Heat Transfer. Thermal Equilibrium. Thermal Inequilibrium
 Heat Transfer, Radiation Convection and Heat Transfer Heat is a form of energy. Heat travels from higher temperature(hotter) region to lower temperature(cooler) region. Two bodies are in thermal equilibrium
Heat Transfer, Radiation Convection and Heat Transfer Heat is a form of energy. Heat travels from higher temperature(hotter) region to lower temperature(cooler) region. Two bodies are in thermal equilibrium
Heat Transfer and Your Electric Bill
 efinitions of Energy Heat Transfer and Your Electric ill (Lexile 740L) 1 Summer is hot in most parts of Texas. Temperatures outside can go over 100 F (38 ). fter being outside in this heat, it is so nice
efinitions of Energy Heat Transfer and Your Electric ill (Lexile 740L) 1 Summer is hot in most parts of Texas. Temperatures outside can go over 100 F (38 ). fter being outside in this heat, it is so nice
Fundamentals of Heat Transfer
 Fundamentals of Heat Transfer State basic laws of heat transfer Estimate heat transfer rates by different modes Use the concept of thermal resistance to solve steady state heat transfer problems State
Fundamentals of Heat Transfer State basic laws of heat transfer Estimate heat transfer rates by different modes Use the concept of thermal resistance to solve steady state heat transfer problems State
UNIQUE COLLEGE OF COMPUTER SCIENCES General Certificate of Education Ordinary Level
 UNIQUE OLLEGE OF OMPUTER SIENES General ertificate of Education Ordinary Level PHYSIS Paper 1 Multiple hoice lass II2 III2 995054/11 January 2011 91 hour dditional Materials: Multiple hoice nswer Sheet
UNIQUE OLLEGE OF OMPUTER SIENES General ertificate of Education Ordinary Level PHYSIS Paper 1 Multiple hoice lass II2 III2 995054/11 January 2011 91 hour dditional Materials: Multiple hoice nswer Sheet
Transfer of Heat. There are three ways in which heat is transferred from one body to another. These are
 Transfer of Heat In the precious chapter you have learnt that heat flows from a body at a higher temperature to a body at a lower temperature, but how does this transfer take place? You will find the answer
Transfer of Heat In the precious chapter you have learnt that heat flows from a body at a higher temperature to a body at a lower temperature, but how does this transfer take place? You will find the answer
S2 Science. Heat. Homework Booklet
 S2 Science Heat Homework Booklet 1 1. The graph below shows how most substances cool after being taken away from the source of heat. Answer these questions about the graph a. where is the heat loss the
S2 Science Heat Homework Booklet 1 1. The graph below shows how most substances cool after being taken away from the source of heat. Answer these questions about the graph a. where is the heat loss the
Heat and temperature. Making a thermometer
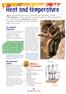 Heat and temperature A bout four hundred years ago, it would have been impossible to tell the temperature of the air, the water or any other substance. That s because there was no such thing as a thermometer
Heat and temperature A bout four hundred years ago, it would have been impossible to tell the temperature of the air, the water or any other substance. That s because there was no such thing as a thermometer
IGCSE PHYSICS GRADE 11 TERM 1 ASSESSMENT BOOKLET
 PHYSICS IGCSE PHYSICS GRADE 11 TERM 1 ASSESSMENT BOOKLET 2013-2014 STS Page 1 of 44 PHYSICS PHYSI1101 ASSESSMENT TASK COVER PAGE Topic STS Performance Criteria Assessment event Date Time Thermal Physics
PHYSICS IGCSE PHYSICS GRADE 11 TERM 1 ASSESSMENT BOOKLET 2013-2014 STS Page 1 of 44 PHYSICS PHYSI1101 ASSESSMENT TASK COVER PAGE Topic STS Performance Criteria Assessment event Date Time Thermal Physics
7 In the process of convection, heat energy is transferred C D E. 9 Boiling water and ice can exist at the same time in a test tube.
 TOPI 9 Transfer of Thermal Energy 1 How may heat be transferred through a vacuum? by convection only by radiation only by conduction only by convection and radiation only E by conduction. convection and
TOPI 9 Transfer of Thermal Energy 1 How may heat be transferred through a vacuum? by convection only by radiation only by conduction only by convection and radiation only E by conduction. convection and
Energy Conservation. Meet Mr.A and Mr.B. They have both received their electricity bill. One of them is happy and one of them is not.
 Name: Class: Date: Grade 11A Science Related Reading/Physics Energy Conservation Physical Processes 11A PRE READING TASK Meet Mr.A and Mr.B. They have both received their electricity bill. One of them
Name: Class: Date: Grade 11A Science Related Reading/Physics Energy Conservation Physical Processes 11A PRE READING TASK Meet Mr.A and Mr.B. They have both received their electricity bill. One of them
Particle Model Questions 1
 Particle Model Questions 35 Questions Name: Class: Date: Time: Marks: Comment s: Q. Figure shows solid ice on a car s rear window. Figure Captive cookies/istock/thinkstock The glass window contains an
Particle Model Questions 35 Questions Name: Class: Date: Time: Marks: Comment s: Q. Figure shows solid ice on a car s rear window. Figure Captive cookies/istock/thinkstock The glass window contains an
Trade of Plumbing. Module 2: Domestic Hot and Cold Water Service Unit 11: Sanitary Appliances Phase 2
 Trade of Plumbing Module 2: Domestic Hot and Cold Water Service Unit 11: Sanitary Appliances Phase 2 Table of Contents List of Figures... 4 List of Tables... 5 Document Release History... 6 Module 2 Domestic
Trade of Plumbing Module 2: Domestic Hot and Cold Water Service Unit 11: Sanitary Appliances Phase 2 Table of Contents List of Figures... 4 List of Tables... 5 Document Release History... 6 Module 2 Domestic
St. Anthony's Canossian Secondary School Sec 3E Science (Physics) Chapter 9 Transfer of Thermal Energy. Name: ( ) Class: Sec Date:
 St. Anthony's Canossian Secondary School Sec 3E Science (Physics) Chapter 9 Transfer of Thermal Energy Name: ( ) Class: Sec Date: Candidates should be able to: (a) show understanding that thermal energy
St. Anthony's Canossian Secondary School Sec 3E Science (Physics) Chapter 9 Transfer of Thermal Energy Name: ( ) Class: Sec Date: Candidates should be able to: (a) show understanding that thermal energy
Thermal Energy Worksheets
 Thermal Energy Worksheets Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
Thermal Energy Worksheets Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit
liquid heating The density of the liquid changes as its temperature increases. This causes energy to be transferred throughout the liquid.
 1 liquid is heated in a beaker. liquid heating The density of the liquid changes as its temperature increases. This causes energy to be transferred throughout the liquid. How does the density change and
1 liquid is heated in a beaker. liquid heating The density of the liquid changes as its temperature increases. This causes energy to be transferred throughout the liquid. How does the density change and
I. C O N T E N T S T A N D A R D S
 Introductory Physics, High School Learning Standards for a Full First-Year Course I. C O N T E N T S T A N D A R D S and radiation between objects or regions that are at different temperatures. 3.1 Explain
Introductory Physics, High School Learning Standards for a Full First-Year Course I. C O N T E N T S T A N D A R D S and radiation between objects or regions that are at different temperatures. 3.1 Explain
REGISTRATION EXAMINATION, JUNE 2015 LICENSED PLUMBER QUESTION AND ANSWER BOOKLET. Time allowed THREE hours
 Affix label with Candidate Code Number here. If no label, enter candidate Number if known No. 99 REGISTRATION EXAMINATION, JUNE 05 LICENSED PLUMBER INSTRUCTIONS QUESTION AND ANSWER BOOKLET Time allowed
Affix label with Candidate Code Number here. If no label, enter candidate Number if known No. 99 REGISTRATION EXAMINATION, JUNE 05 LICENSED PLUMBER INSTRUCTIONS QUESTION AND ANSWER BOOKLET Time allowed
Science 7 Chapter 6 Section 1
 Science 7 Chapter 6 Section 1 Processes of Transferring Heat Processes of Heat Transfer There are 3 processes responsible for heat transfer: 1) Conduction 2) Convection 3) Radiation 1 Conduction Conduction:
Science 7 Chapter 6 Section 1 Processes of Transferring Heat Processes of Heat Transfer There are 3 processes responsible for heat transfer: 1) Conduction 2) Convection 3) Radiation 1 Conduction Conduction:
In Chapter 3 you learnt that woollen
 4 Heat In Chapter 3 you learnt that woollen clothes are made from animal fibres. You also know that cotton clothes are made from plant fibres. We wear woollen clothes during winters when it is cold outside.
4 Heat In Chapter 3 you learnt that woollen clothes are made from animal fibres. You also know that cotton clothes are made from plant fibres. We wear woollen clothes during winters when it is cold outside.
REGISTRATION EXAMINATION, NOVEMBER 2017 TRADESMAN PLUMBER QUESTION AND ANSWER BOOKLET. Time allowed THREE hours
 Affix label with Candidate Code Number here. If no label, enter candidate Number if known No. 99 REGISTRATION EXAMINATION, NOVEMBER 07 TRADESMAN PLUMBER INSTRUCTIONS QUESTION AND ANSWER BOOKLET Time allowed
Affix label with Candidate Code Number here. If no label, enter candidate Number if known No. 99 REGISTRATION EXAMINATION, NOVEMBER 07 TRADESMAN PLUMBER INSTRUCTIONS QUESTION AND ANSWER BOOKLET Time allowed
P1.1H Infrared Radiation Questions Higher
 P1.1H Infrared Radiation Questions Higher 25 minutes 25 marks Page 1 of 11 Q1. A wood burning stove is used to heat a room. Photograph supplied by istockphoto/thinkstock The fire in the stove uses wood
P1.1H Infrared Radiation Questions Higher 25 minutes 25 marks Page 1 of 11 Q1. A wood burning stove is used to heat a room. Photograph supplied by istockphoto/thinkstock The fire in the stove uses wood
Activity Heat Transfer
 Name: Hr: Activity 3.3.6 Heat Transfer Introduction Heat is the transfer of thermal energy. Thermal energy exists when the atoms or molecules in a substance are in motion and vibrate. The atoms possess
Name: Hr: Activity 3.3.6 Heat Transfer Introduction Heat is the transfer of thermal energy. Thermal energy exists when the atoms or molecules in a substance are in motion and vibrate. The atoms possess
ST. GABRIEL S SECONDARY SCHOOL Lower Secondary Science Chapter 7 Transfer of Thermal Energy
 ST. GRIEL S SEONRY SHOOL Lower Secondary Science hapter 7 Transfer of Thermal Energy Worksheet 7.1 Name : _Suggested nswers ( ) lass : ate : Section : Multiple hoice Questions 1 Two similarly-sized blocks,
ST. GRIEL S SEONRY SHOOL Lower Secondary Science hapter 7 Transfer of Thermal Energy Worksheet 7.1 Name : _Suggested nswers ( ) lass : ate : Section : Multiple hoice Questions 1 Two similarly-sized blocks,
5. Transfer of thermal energy
 5. Transfer of thermal energy A bird can reduce the heat loss from its body during cold weather by fluffing up its feathers. What are the processes of heat transfer? Conduction Convection Radiation transfer
5. Transfer of thermal energy A bird can reduce the heat loss from its body during cold weather by fluffing up its feathers. What are the processes of heat transfer? Conduction Convection Radiation transfer
Four of the appliances, including the fan heater, are designed to transform electrical energy into heat.
 Q1. The pictures show six different household appliances. (a) Four of the appliances, including the fan heater, are designed to transform electrical energy into heat. Name the other three appliances designed
Q1. The pictures show six different household appliances. (a) Four of the appliances, including the fan heater, are designed to transform electrical energy into heat. Name the other three appliances designed
Development of Boiler Design
 Development of Boiler Design Learning Outcome When you complete this module you will be able to: Discuss historical developments of, and the general requirements for proper boiler design. Learning Objectives
Development of Boiler Design Learning Outcome When you complete this module you will be able to: Discuss historical developments of, and the general requirements for proper boiler design. Learning Objectives
3B Heat, Light and Sound
 Heat, Light and Sound Heat, light and sound are forms of energy that have many applications in our lives. Heat Heat is a form of energy When petrol burns in a car engine it causes the gases in the cylinders
Heat, Light and Sound Heat, light and sound are forms of energy that have many applications in our lives. Heat Heat is a form of energy When petrol burns in a car engine it causes the gases in the cylinders
PHYSICS FORM 5 TRANSFER OF THERMAL ENERGY
 Heat energy is transferred from one place to the next by three mechanisms: 1. Conduction 2. Convection 3. Radiation Conduction This is the process of heat transfer from one place to another using the movement/vibration
Heat energy is transferred from one place to the next by three mechanisms: 1. Conduction 2. Convection 3. Radiation Conduction This is the process of heat transfer from one place to another using the movement/vibration
Making a Carousel Lantern. Grade 7 Activity Plan
 Making a Carousel Lantern Grade 7 Activity Plan 1 Reviews and Updates - Carousel Lanterns Activity added by Fola Akpan in July 2017 2 Making a Carousel Lantern Objectives: 1. To compare two methods of
Making a Carousel Lantern Grade 7 Activity Plan 1 Reviews and Updates - Carousel Lanterns Activity added by Fola Akpan in July 2017 2 Making a Carousel Lantern Objectives: 1. To compare two methods of
St. Anthony's Canossian Secondary School Sec 3NA Science (Physics) Chapter 7 Transfer of Thermal Energy. Name: ( ) Class: Sec Date:
 St. Anthony's Canossian Secondary School Sec 3NA Science (Physics) Chapter 7 Transfer of Thermal Energy Name: ( ) Class: Sec Date: Candidates should be able to: (a) show understanding that thermal energy
St. Anthony's Canossian Secondary School Sec 3NA Science (Physics) Chapter 7 Transfer of Thermal Energy Name: ( ) Class: Sec Date: Candidates should be able to: (a) show understanding that thermal energy
Technical Document Condensing hot water boilers
 Condensing hot water boilers Key subjects in selecting a condensing hot water boiler. Efficiency Material of construction Design of construction Control capabilities Turndown Flow rate Installation Warranty
Condensing hot water boilers Key subjects in selecting a condensing hot water boiler. Efficiency Material of construction Design of construction Control capabilities Turndown Flow rate Installation Warranty
Cork Institute of Technology Higher Certificate in Engineering in Building Services Engineering Award
 Cork Institute of Technology Higher Certificate in Engineering in Building Services Engineering Award Instructions Answer FIVE questions, All questions carry equal marks. (NFQ Level 6) Autumn 2006 Building
Cork Institute of Technology Higher Certificate in Engineering in Building Services Engineering Award Instructions Answer FIVE questions, All questions carry equal marks. (NFQ Level 6) Autumn 2006 Building
Page 22a. What heats up faster, sand or water? Which one has a greater specific heat capacity?
 Page 22a Have weather map face-up on table Objective: We will describe the three types of heat transfer and explain their roles in Earth processes. Warm-up: What heats up faster, sand or water? Which one
Page 22a Have weather map face-up on table Objective: We will describe the three types of heat transfer and explain their roles in Earth processes. Warm-up: What heats up faster, sand or water? Which one
Estimate the energy stored in unit gram of the food in J per gram.
 NAME : F.5 ( ) Marks: /70 FORM FIVE PHYSICS REVISION TEST on HEAT Time Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section
NAME : F.5 ( ) Marks: /70 FORM FIVE PHYSICS REVISION TEST on HEAT Time Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section
AS Australian Standard. Domestic solid fuel burning appliances Installation. This is a free 6 page sample. Access the full version online.
 AS 2918 1990 Australian Standard Domestic solid fuel burning appliances Installation This Australian Standard was prepared by Committee CS/62, Solid Fuel Burning Appliances. It was approved on behalf of
AS 2918 1990 Australian Standard Domestic solid fuel burning appliances Installation This Australian Standard was prepared by Committee CS/62, Solid Fuel Burning Appliances. It was approved on behalf of
Use numbers given in the box to complete the following sentences. In the UK, the mains electricity supply is volts.
 DOMESTIC USES AND SAFETY PART II Q1. Use numbers given in the box to complete the following sentences. 12 50 110 230 In the UK, the mains electricity supply is volts. The frequency of the UK mains electricity
DOMESTIC USES AND SAFETY PART II Q1. Use numbers given in the box to complete the following sentences. 12 50 110 230 In the UK, the mains electricity supply is volts. The frequency of the UK mains electricity
2018 Year 11 Physics Week 8. Thermal Energy Transfer
 2018 Year 11 Physics Week 8 Thermal Energy Transfer Thermal energy Thermal or heat energy is the energy that flows from a hot region to a cold region by one or more of the processes of: CONDUCTION CONVECTION
2018 Year 11 Physics Week 8 Thermal Energy Transfer Thermal energy Thermal or heat energy is the energy that flows from a hot region to a cold region by one or more of the processes of: CONDUCTION CONVECTION
More heat energy means more of what type of energy? Does the mass change? So, what must change? What is the same in both containers?
 Quest Chapter 21-23 # Problem Hint 1 When a container of gas is heated, what happens to the average speed of its molecules? 1. Additional information is needed. 2. increases 3. doesn t change 4. decreases
Quest Chapter 21-23 # Problem Hint 1 When a container of gas is heated, what happens to the average speed of its molecules? 1. Additional information is needed. 2. increases 3. doesn t change 4. decreases
Transfer of Thermal Energy
 Transfer of Thermal Energy Transfer of Thermal Energy Thermal Energy Transfer Conduction Convection Radiation Transfer of Thermal Energy Thermal Energy Transfer Conduction Convection Radiation Lesson Objectives
Transfer of Thermal Energy Transfer of Thermal Energy Thermal Energy Transfer Conduction Convection Radiation Transfer of Thermal Energy Thermal Energy Transfer Conduction Convection Radiation Lesson Objectives
UNIQUE SCIENCE ACADEMY
 UNIQUE SIENE EMY Test (Unit 11-12) Name :... Paper: Physics ate :... ode: 5054 lass: II Time llowed: 35Minutes This document consists of 5 printed pages. Maximum Marks: 25 T [Total 21 Marks] heory Section:
UNIQUE SIENE EMY Test (Unit 11-12) Name :... Paper: Physics ate :... ode: 5054 lass: II Time llowed: 35Minutes This document consists of 5 printed pages. Maximum Marks: 25 T [Total 21 Marks] heory Section:
Level 1 Physics, 2016
 90939 909390 1SUPERVISOR S Level 1 Physics, 2016 90939 Demonstrate understanding of aspects of heat 2.00 p.m. Tuesday 15 November 2016 Credits: Four Achievement Achievement with Merit Achievement with
90939 909390 1SUPERVISOR S Level 1 Physics, 2016 90939 Demonstrate understanding of aspects of heat 2.00 p.m. Tuesday 15 November 2016 Credits: Four Achievement Achievement with Merit Achievement with
ASBESTOS ADVICE FOR RESIDENTS
 ASBESTOS ADVICE FOR RESIDENTS This leaflet provides information about asbestos in the home and within shared or communal areas. It tells you what asbestos is, where you might find it, why it may be a
ASBESTOS ADVICE FOR RESIDENTS This leaflet provides information about asbestos in the home and within shared or communal areas. It tells you what asbestos is, where you might find it, why it may be a
Use words from the list to complete the following sentences. Words may be used once, more than once, or not at all.
 Q1. The diagram shows an experimental solar-powered bike. A battery is connected to the solar cells. The solar cells charge up the battery. There is a switch on the handlebars. When the switch is closed,
Q1. The diagram shows an experimental solar-powered bike. A battery is connected to the solar cells. The solar cells charge up the battery. There is a switch on the handlebars. When the switch is closed,
Weather: Why do we have weather? Does it serve a purpose?
 Weather: Why do we have weather? Does it serve a purpose? Heat, insulation, the Earth and you: Why people wear different colored clothing in different parts of the world Heat Part I Grandpa, why do people
Weather: Why do we have weather? Does it serve a purpose? Heat, insulation, the Earth and you: Why people wear different colored clothing in different parts of the world Heat Part I Grandpa, why do people
6. Within an internal combustion engine, the can-shaped component that moves up and down the cylinder
 Student ID: 22133336 Exam: 986052RR - Heat When you have completed your exam and reviewed your answers, click Submit Exam. Answers will not be recorded until you hit Submit Exam. If you need to exit before
Student ID: 22133336 Exam: 986052RR - Heat When you have completed your exam and reviewed your answers, click Submit Exam. Answers will not be recorded until you hit Submit Exam. If you need to exit before
Energy Saving Series RADICAL
 RADICAL The Stelrad Radical Energy Saving Radiator can save up to 10.5% on energy bills, delivering higher comfort levels at a lower thermostat setting. That s radical thinking, from Stelrad. It s the
RADICAL The Stelrad Radical Energy Saving Radiator can save up to 10.5% on energy bills, delivering higher comfort levels at a lower thermostat setting. That s radical thinking, from Stelrad. It s the
VISUAL PHYSICS ONLINE THERMODYNAMICS WHAT HAPPENS WHEN SOMETHING IS HEATED? LATENT HEAT
 VISUAL PHYSICS ONLINE THERMODYNAMICS WHAT HAPPENS WHEN SOMETHING IS HEATED? LATENT HEAT 1 HEATS OF FUSION AND VAPORISATION From the equation containing the specific heat, it seems that we can keep on transferring
VISUAL PHYSICS ONLINE THERMODYNAMICS WHAT HAPPENS WHEN SOMETHING IS HEATED? LATENT HEAT 1 HEATS OF FUSION AND VAPORISATION From the equation containing the specific heat, it seems that we can keep on transferring
Heat Energy. Heat Energy. A Science A Z Physical Series. Word Count: 1,301. Written by Felicia Brown. Visit
 Heat Energy A Science A Z Physical Series Word Count: 1,301 Heat Energy Written by Felicia Brown Visit www.sciencea-z.com www.sciencea-z.com Heat Energy Key elements Used in This Book The Big Idea: One
Heat Energy A Science A Z Physical Series Word Count: 1,301 Heat Energy Written by Felicia Brown Visit www.sciencea-z.com www.sciencea-z.com Heat Energy Key elements Used in This Book The Big Idea: One
Q1. The diagram shows the design of a solar cooker. The cooker heats water using infrared radiation from the Sun.
 Q. The diagram shows the design of a solar cooker. The cooker heats water using infrared radiation from the Sun. (a) Why is the inside of the large curved dish covered with shiny metal foil? () (b) Which
Q. The diagram shows the design of a solar cooker. The cooker heats water using infrared radiation from the Sun. (a) Why is the inside of the large curved dish covered with shiny metal foil? () (b) Which
Unit 1: Fire Engineering Science (A/505/6005)
 L3D1 THE INSTITUTION OF FIRE ENGINEERS Founded 1918 Incorporated 1924 IFE Level 3 Diploma in Fire Science and Fire Safety (VRQ) Unit 1: Fire Engineering Science (A/505/6005) Friday 11 March 2016 10.30
L3D1 THE INSTITUTION OF FIRE ENGINEERS Founded 1918 Incorporated 1924 IFE Level 3 Diploma in Fire Science and Fire Safety (VRQ) Unit 1: Fire Engineering Science (A/505/6005) Friday 11 March 2016 10.30
REGISTRATION EXAMINATION, NOVEMBER 2014 LICENSED PLUMBER QUESTION AND ANSWER BOOKLET. Time allowed THREE hours
 Affix label with Candidate Code Number here. If no label, enter candidate Number if known No. 9192 REGISTRATION EXAMINATION, NOVEMBER 2014 LICENSED PLUMBER INSTRUCTIONS QUESTION AND ANSWER BOOKLET Time
Affix label with Candidate Code Number here. If no label, enter candidate Number if known No. 9192 REGISTRATION EXAMINATION, NOVEMBER 2014 LICENSED PLUMBER INSTRUCTIONS QUESTION AND ANSWER BOOKLET Time
THERMAL CONDUCTION. placed in a different position. Can you explain why the matches go out?
 THERMAL CONDUCTION NAME(S) Pour about 250 ml of water into a 500 ml beaker, and begin heating the beaker on a hot plate. The beaker of water will be used in a later activity. Activity #1 A Parlor Trick
THERMAL CONDUCTION NAME(S) Pour about 250 ml of water into a 500 ml beaker, and begin heating the beaker on a hot plate. The beaker of water will be used in a later activity. Activity #1 A Parlor Trick
M1. (a) (i) walls accept sides (of house) 1
 M. (a) (i) walls accept sides (of house) fit double glazing or close / fit curtains / fit shutters accept close windows accept keep house at a lower temperature accept fit (foam) draft excluders around
M. (a) (i) walls accept sides (of house) fit double glazing or close / fit curtains / fit shutters accept close windows accept keep house at a lower temperature accept fit (foam) draft excluders around
REGISTRATION EXAMINATION, NOVEMBER 2016 LICENSED GASFITTER QUESTION AND ANSWER BOOKLET. Time allowed THREE hours
 Affix label with Candidate Code Number here. If no label, enter candidate Number if known No. 993 REGISTRATION EXAMINATION, NOVEMBER 06 LICENSED GASFITTER INSTRUCTIONS QUESTION AND ANSWER BOOKLET Time
Affix label with Candidate Code Number here. If no label, enter candidate Number if known No. 993 REGISTRATION EXAMINATION, NOVEMBER 06 LICENSED GASFITTER INSTRUCTIONS QUESTION AND ANSWER BOOKLET Time
Steam: Yesterday, Today and Tomorrow The Use of Steam as an Efficient Heat Source
 Steam: Yesterday, Today and Tomorrow The Use of Steam as an Efficient Heat Source Tech Sheet #ST 103 The mention of steam conjures up the thought of days gone by when steam was used to power engines, to
Steam: Yesterday, Today and Tomorrow The Use of Steam as an Efficient Heat Source Tech Sheet #ST 103 The mention of steam conjures up the thought of days gone by when steam was used to power engines, to
INCREASING BOILER EFFICIENCY BY USING DIFFERENT MEASUREMENTS AND TYPES OF FIRETUBE PLATES AND DIFFERENT NOZZLES
 INCREASING BOILER EFFICIENCY BY USING DIFFERENT MEASUREMENTS AND TYPES OF FIRETUBE PLATES AND DIFFERENT NOZZLES Bassam A. Al-Helou Mechanical Engineering Department Faculty of Engineering Technology -
INCREASING BOILER EFFICIENCY BY USING DIFFERENT MEASUREMENTS AND TYPES OF FIRETUBE PLATES AND DIFFERENT NOZZLES Bassam A. Al-Helou Mechanical Engineering Department Faculty of Engineering Technology -
Steam The Primary Force
 HOW POWER PLANTS WORK Lesson One Steam The Primary Force 11101 4 Lesson 1 Steam The Primary Force TOPICS Energy for Power Plants Converting Energy to Electricity The Importance of Air in Combustion Removing
HOW POWER PLANTS WORK Lesson One Steam The Primary Force 11101 4 Lesson 1 Steam The Primary Force TOPICS Energy for Power Plants Converting Energy to Electricity The Importance of Air in Combustion Removing
Describe the movement of the particles of helium gas inside the balloon
 Q1.The figure below shows a balloon filled with helium gas. (a) Describe the movement of the particles of helium gas inside the balloon............. (b) What name is given to the total kinetic energy and
Q1.The figure below shows a balloon filled with helium gas. (a) Describe the movement of the particles of helium gas inside the balloon............. (b) What name is given to the total kinetic energy and
Radical CPD BIM.
 Radical At the forefront of innovation, Stelrad has developed the next generation in energy saving radiators, the Radical. As the first serial feed radiator this ground breaking solution reduces energy
Radical At the forefront of innovation, Stelrad has developed the next generation in energy saving radiators, the Radical. As the first serial feed radiator this ground breaking solution reduces energy
The Ultimate Solution for Electric Central Heating. Fusion. Comet
 The Ultimate Solution for Electric Central Heating Fusion Comet Our range of Electric Boilers provide complete peace of mind for reliability, performance and safety 2 The Fusion range of electric boilers
The Ultimate Solution for Electric Central Heating Fusion Comet Our range of Electric Boilers provide complete peace of mind for reliability, performance and safety 2 The Fusion range of electric boilers
LESSON CLUSTER 9 Explaining Condensation and the Water Cycle
 LESSON CLUSTER 9 Explaining Condensation and the Water Cycle Lesson 9.1: Boiling and Condensation You have been studying changes of state for quite a while now. You have studied melting, freezing or solidifying,
LESSON CLUSTER 9 Explaining Condensation and the Water Cycle Lesson 9.1: Boiling and Condensation You have been studying changes of state for quite a while now. You have studied melting, freezing or solidifying,
Steam and Gas Power Systems Prof Ravi Kumar Department of Mechanical and Industrial Engineering Indian Institute of Technology - Roorkee
 Steam and Gas Power Systems Prof Ravi Kumar Department of Mechanical and Industrial Engineering Indian Institute of Technology - Roorkee Module No # 02 Lecture No # 09 Boilers Mountings and Accessories
Steam and Gas Power Systems Prof Ravi Kumar Department of Mechanical and Industrial Engineering Indian Institute of Technology - Roorkee Module No # 02 Lecture No # 09 Boilers Mountings and Accessories
 RAYBURN SUPREME/NOUVELLE/355S USER INSTRUCTIONS ISSUE 4 Water Temperature Control Stat. http://www.oilstoves.co.uk/ 1 2 Contents 1. Introduction. 1 2. How Does it Operate? 1 3. Description of Controls.
RAYBURN SUPREME/NOUVELLE/355S USER INSTRUCTIONS ISSUE 4 Water Temperature Control Stat. http://www.oilstoves.co.uk/ 1 2 Contents 1. Introduction. 1 2. How Does it Operate? 1 3. Description of Controls.
Level 6 Using Physics: Investigate how physics knowledge is used in a technological application.
 WHAT S COOKING WITH SOLAR? TEACHER NOTES Rationale for the Activity The activity enables students to see how scientific concepts are applied in the design and construction of effective technologies, and
WHAT S COOKING WITH SOLAR? TEACHER NOTES Rationale for the Activity The activity enables students to see how scientific concepts are applied in the design and construction of effective technologies, and
Qualification Guide. BPEC 601/2514/7 - Level 1 Diploma in Plumbing Foundation. BPEC 600/9432/1 - Level 2 Diploma in Plumbing Foundation
 Qualification Guide BPEC 601/2514/7 - Level 1 Diploma in Plumbing Foundation BPEC 600/9432/1 - Level 2 Diploma in Plumbing Foundation BPEC 600/5270/3 - Level 2 NVQ Diploma in Plumbing and Heating BPEC
Qualification Guide BPEC 601/2514/7 - Level 1 Diploma in Plumbing Foundation BPEC 600/9432/1 - Level 2 Diploma in Plumbing Foundation BPEC 600/5270/3 - Level 2 NVQ Diploma in Plumbing and Heating BPEC
BASIC BOILERS NAME :- DATE :-
 BASIC BOILERS NAME :- DATE :- This examination was conducted under conditions that allowed a fair and honest test of the candidate s knowledge and ability. Exam supervisor C:\Tassie Steam\N01basicboiler
BASIC BOILERS NAME :- DATE :- This examination was conducted under conditions that allowed a fair and honest test of the candidate s knowledge and ability. Exam supervisor C:\Tassie Steam\N01basicboiler
Everything you need to know about Radiant Heating but didn t dare ask
 Decorative radiators Comfortable indoor ventilation Heating and cooling ceiling systems Clean air solutions Everything you need to know about Radiant Heating but didn t dare ask Introduction Radiant heating
Decorative radiators Comfortable indoor ventilation Heating and cooling ceiling systems Clean air solutions Everything you need to know about Radiant Heating but didn t dare ask Introduction Radiant heating
Thermal Energy Study Guide
 Goal 1 Thermal Energy Study Guide Name: Hour Define the following in your own words: 1. Conductor - a material that allows heat to flow through it easily Two examples - metal pot, fork 2. Insulator - a
Goal 1 Thermal Energy Study Guide Name: Hour Define the following in your own words: 1. Conductor - a material that allows heat to flow through it easily Two examples - metal pot, fork 2. Insulator - a
Compaction versus the value of airspace: Solid Waste Compaction in Sanitary Landfills
 Compaction versus the value of airspace: Solid Waste Compaction in Sanitary Landfills Author: Stéphane BERTRAND CATERPILLAR Europe, Africa and the Middle East. Industrial and Waste applications specialist
Compaction versus the value of airspace: Solid Waste Compaction in Sanitary Landfills Author: Stéphane BERTRAND CATERPILLAR Europe, Africa and the Middle East. Industrial and Waste applications specialist
Adelaide Homes Design Guide 4 - Winter warming
 Adelaide Homes Design Guide 4 - Winter warming Adelaide's temperate climate means home warming is required during winter. There are many efficient heating systems. The most economical method, however,
Adelaide Homes Design Guide 4 - Winter warming Adelaide's temperate climate means home warming is required during winter. There are many efficient heating systems. The most economical method, however,
MINTRAC MEAT INDUSTRY ENVIRONMENT CONFERENCE WASTE HEAT RECOVERY FROM RENDERING PLANTS PRESENTED BY DEREK HENDERSON
 Keith Engineering (Australia) Pty Ltd ABN 20 109 344 185 20 Kellet Close, Erskine Park, NSW 2759 PO Box 354, St Clair NSW 2759 T +61 2 9852 1000 F +61 2 9852 1001 admin@keitheng.com.au www.keitheng.com.au
Keith Engineering (Australia) Pty Ltd ABN 20 109 344 185 20 Kellet Close, Erskine Park, NSW 2759 PO Box 354, St Clair NSW 2759 T +61 2 9852 1000 F +61 2 9852 1001 admin@keitheng.com.au www.keitheng.com.au
STRESS-FREE RENOVATIONS By Nick Brownlee, Managing Director Procuro Building and Renovation
 STRESS-FREE RENOVATIONS By Nick Brownlee, Managing Director Procuro Building and Renovation BUILDING & RENOVATION RENOVATING YOUR HOUSE INTO YOUR DREAM HOME IS A PRIVILEGE WE ENJOY EVERY DAY. Procuro Building
STRESS-FREE RENOVATIONS By Nick Brownlee, Managing Director Procuro Building and Renovation BUILDING & RENOVATION RENOVATING YOUR HOUSE INTO YOUR DREAM HOME IS A PRIVILEGE WE ENJOY EVERY DAY. Procuro Building
Applications of Thermodynamics: Heat Pumps and Refrigerators
 Applications of Thermodynamics: Heat Pumps and Refrigerators Bởi: OpenStaxCollege Almost every home contains a refrigerator. Most people don t realize they are also sharing their homes with a heat pump.
Applications of Thermodynamics: Heat Pumps and Refrigerators Bởi: OpenStaxCollege Almost every home contains a refrigerator. Most people don t realize they are also sharing their homes with a heat pump.
Operating Performance
 INFORMATION SHEET 1 Jackson Engineering Advisers are specialist Building Services Engineers. This information sheet is offered as an aid to Building Owners and Managers as a guide to plant life expectancies.
INFORMATION SHEET 1 Jackson Engineering Advisers are specialist Building Services Engineers. This information sheet is offered as an aid to Building Owners and Managers as a guide to plant life expectancies.
Changes of phase usually involve a transfer of energy Evaporation
 Changes of phase usually involve a transfer of energy. The four possible forms of matter solid, liquid, gas, and plasma are called phases. Matter can change from one phase to another. The phase of matter
Changes of phase usually involve a transfer of energy. The four possible forms of matter solid, liquid, gas, and plasma are called phases. Matter can change from one phase to another. The phase of matter
PLUMBING CURRICULA OUTLINE
 PLUMBING CURRICULA OUTLINE CORE CURRICULUM 2015 Basic Safety (Construction Site Safety Orientation) (12.5 Hours) (Module ID 00101-15) Presents basic jobsite safety information to prepare workers for the
PLUMBING CURRICULA OUTLINE CORE CURRICULUM 2015 Basic Safety (Construction Site Safety Orientation) (12.5 Hours) (Module ID 00101-15) Presents basic jobsite safety information to prepare workers for the
EASY$ TIP SHEETS. Energy Advice Saving Yukoners Money. How hydronic heating systems work
 EASY$ TIP SHEETS Energy Advice Saving Yukoners Money Quick Links How hydronic heating systems work Advantages Disadvantages Design alternatives Baseboard convectors Residential Hydronic Heating A residential
EASY$ TIP SHEETS Energy Advice Saving Yukoners Money Quick Links How hydronic heating systems work Advantages Disadvantages Design alternatives Baseboard convectors Residential Hydronic Heating A residential
Within the context of a camping scenario, students are asked to apply their understanding of the principles of heat transfer to various situations.
 Sample assessment task Year level 9 Learning area Subject Title of task Task details of task Type of assessment Suggested time Science Physical Sciences Camping scenario and heat Within the context of
Sample assessment task Year level 9 Learning area Subject Title of task Task details of task Type of assessment Suggested time Science Physical Sciences Camping scenario and heat Within the context of
Physics. Mr Rishi Gopie THERMAL ENERGY TRANSFER
 Mr Rishi Gopie THERMAL ENERGY TRANSFER Thermal Energy Transfer Thermal energy transferred from one system/region to another due to a temperature difference is called heat and there are three main ways
Mr Rishi Gopie THERMAL ENERGY TRANSFER Thermal Energy Transfer Thermal energy transferred from one system/region to another due to a temperature difference is called heat and there are three main ways
